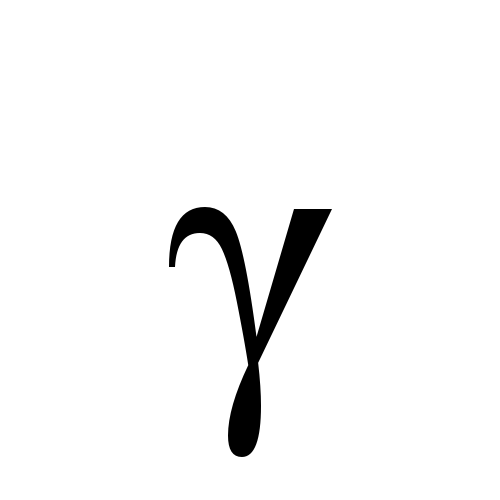August 2025: Diagnosing (disease-related) malnutrition in patients with obesity is challenging due to the complex interplay between excess body weight and physiological changes associated with illness and inadequate dietary intake, factors often overlooked in clinical assessments. Current global definitions of malnutrition do not adequately account for the distinctive characteristics of patients with obesity. This study aimed to develop a working definition of malnutrition in this population. A modified three-round Delphi method was conducted between March and July 2024, involving 25 experts to achieve consensus on diagnosing malnutrition in obesity. In Round 1, participants evaluated 45 statements using a 5-point Likert scale. Feedback from this round guided revisions for Round 2, which focused on the Global Leadership Initiative on Malnutrition (GLIM) criteria and introduced nine revised statements. Round 3 further refined these statements, with the final consensus assessed using a binary agree/disagree scale. A threshold of ≥70 % agreement was set to define consensus in all rounds, with statements not meeting this threshold left undecided. Participation rates were 88 % (n = 22) in Round 1, 77 % (n = 17) in Round 2, and 50 % (n = 11) in Round 3. Of the 45 statements assessed in Round 1, 11 were accepted, 32 were undecided, and two were rejected. Round 2 introduced nine revised statements, of which seven were accepted and two remained undecided. In Round 3, nine statements were assessed, of which six were accepted, and three remained undecided. Consensus supported adopting the GLIM criteria as the foundation for the working definition. However, thresholds for weight loss and muscle mass and the relevance of functional parameters remained unresolved. C-reactive protein thresholds were agreed upon, but their relevance was debated due to the challenges in interpreting chronic low-grade inflammation in obesity. Participants emphasised the importance of assessing dietary quality and quantity, recommending dietitian involvement for improved accuracy. Although a working definition for diagnosing malnutrition in patients with obesity was not achieved, this study lays a crucial foundation for further research. Key areas for future investigation include refining and validating parameters related to involuntary weight loss, muscle mass, inflammatory markers and dietary intake. Natasha Nalucha Mwala wrote the paper. An additional corrigendum here.
July 2025: The metabolic syndrome (MetS) is a growing health issue. This study evaluated the prevalence of MetS, and individual MetS risk factors, in people with different neuromuscular diseases (NMD). We used baseline data of a randomized controlled trial on the efficacy of a physical activity program in NMD. MetS was defined as the presence of at least 3 out of 5 risk factors of the revised National Cholesterol Education Program Adult Treatment Panel III. The 84 participants (50 female, median age = 63 years, IQR = 48, 68 years) were diagnosed with post-polio syndrome (PPS, n = 22), Charcot-Marie-Tooth disease (CMT, n = 37) or other NMD (n = 25). MetS was present in 18 participants (21%). The most common risk factors were hypertension (56%), central obesity (49%) and increased fasting blood glucose (33%). Logistic regression results showed that, adjusted for age and muscle strength as confounders, participants with CMT (Exp(B) = 0.107, 95%CI: 0.019-0.609) and other NMD (Exp(B) = 0.039, 95%CI: 0.004-0.390) had significantly lower odds of MetS compared with PPS. The MetS prevalence that we found is comparable to the general Dutch population. However, a focus on the prevention of MetS in neuromuscular rehabilitation is warranted, as certain NMD subgroups may be at increased risk of developing MetS. The paper was written by Eric Voorn.
July 2025: To improve global- and environmental health, the Dutch Green Deal Sustainable Healthcare (DGD) guidelines recommend to replace at least 50 % of animal protein with plant-derived protein. This may be a challenge for hospitalized patients due to the low protein content and the lack of Essential Amino Acids (EAA) in individual plant-derived sources in combination with anabolic resistance during disease. Yet, there is little knowledge about the effect on protein- and amino acid intake among hospitalized patients as we shift to more plant-derived diets. Therefore, this observational study examines (plant- and animal) protein intake and Amino Acid Scores (AAS) of predominantly plant- and animal derived meals in a large university hospital. Food intake data were collected through direct observation in non-critically ill adult patients between October and November 2023. Protein requirements were set on 1.2 g/kg body weight, adjusted for BMI. For data analysis, patients were divided into three groups based on their total protein intake: low (<0.8 g/kg), moderate (0.8-1.1 g/kg) and adequate (≥1.2 g/kg). Meals were considered predominantly plant-derived if plant protein (in grams) accounted for over 50 % of its total protein content. AAS were determined per meal by assessing the amount of EAA per gram of protein relative to EAA requirements. In total, 234 patients were included. Protein intake was insufficient in 80 % of all patients. The overall animal-to plant protein ratio was 69:31. Among patients who consumed more than 50 % plant-derived protein per meal, lysine was the most common limiting amino acid (AAS <1). In contrast, no limiting AAS per gram of protein were found for patients consuming more than 50 % animal-derived protein per meal. Achieving sufficient protein intake (1.2 g/kg) is a key challenge especially in the shift towards more plant-derived nutrition. Although the predominantly plant-derived meals require careful attention to amino acid profiles, especially for lysine, the low total protein content of predominantly plant-derived meals poses the greatest challenge, thereby limiting the feasibility of the protein transition for hospitalized patients. Maaike van Bree did all the work!
May 2025: Inflammation, oxidative stress, and bioenergetic dysfunction are proposed underlying mechanisms of schizophrenia spectrum disorders (SSDs) and bipolar disorders (BDs), contributing to the largely untreated cognitive and negative symptoms in these conditions. Ketone bodies may offer a therapeutic option for these symptoms through their positive effects on the aforementioned mechanisms. Exogenous ketones like ketone esters (KEs) provide a means to quickly induce ketosis without dietary restrictions, though their effects on SSD and BD have not yet been investigated. This ongoing triple-blind, randomized controlled crossover trial investigates the effects of a single ingestion of KE on signs and symptoms of SSD and BD. A total of 24 patients (12 SSD and 12 BD) receiving inpatient care at Amsterdam University Medical Center (UMC) will be included in the study. Patients will ingest a single dose of KE ((R)-3-hydroxybutyl-(R)-3-hydroxybutyrate deltaG Ketones (dGK) and an isocaloric carbohydrate control with a washout period of 3 days between drinks. The primary outcome is the change in prepulse inhibition of the startle reflex induced by dGK ingestion compared with control. Secondary outcomes include resting-state electroencephalography, P3B amplitude, cognitive performance, and metabolic, immune, oxidative stress, and circadian rhythm parameters. Feasibility and potential side effects will also be assessed. Our current study will offer valuable preliminary data on the effects of KE in patients with SSD and BD. It can provide the foundation for future research into the therapeutic potential of KE in alleviating symptoms and improving functional outcomes in these disorders.This trial was registered at www.clinicaltrials.gov as NCT06426134. Daphne Dielemans and Karin Huizer wrote this paper!
May 2025: Restrictive anorexia nervosa (AN-R) is characterized not only by psychiatric manifestations but also by significant medical complications. Patients commonly exhibit immune alterations, potentially increasing their susceptibility to infections. While direct evidence linking AN-R to heightened rates of opportunistic infections remains inconclusive, clinical observations suggest a higher incidence of complications and delayed febrile response in patients with infections. Concurrently, malnutrition, a frequent cause of secondary immunodeficiencies, exacerbates this susceptibility by compromising immune function. This paper investigates the immunological profiles of two patients with long-term AN-R who developed severe infections: one with disseminated Mycobacterium kansasii and the other with a co-infection of pulmonary Aspergillus fumigatus and Mycobacterium celatum. These cases, alongside data collected from previously published case reports summarized in this study, highlight the impact of altered immune function associated with mentioned population. The paper aims to explain the underlying mechanisms of immune dysfunction. Proactive monitoring of immune status and incorporating such assays into clinical practice may benefit early detection, effective management, and ultimately, improved outcomes. Petra Vidmar first-authored this paper!
April 2025: Bile acids play vital roles in control of lipid, glucose, and energy metabolism by activating Takeda G protein-coupled receptor 5 and Farnesoid X receptor, the latter promoting production of the endocrine-acting fibroblast growth factor 19 (FGF19). Short-term administration of single bile acids has been reported to enhance plasma levels of GLP-1 and to enhance energy expenditure. However, prolonged bile acid supplementation (eg, of chenodeoxycholic acid for gallstone dissolution) has been reported to have adverse effects. In this proof-of-concept study, we assessed the safety and metabolic effects of oral glycine-conjugated deoxycholic acid (GDCA) administration at 10 mg/kg/day using regular and slow-release capsules (mimicking physiological bile acid release) over 30 days in 2 groups of each 10 healthy lean men, respectively. GDCA increased postprandial total bile acid and FGF19 concentrations while suppressing those of the primary bile acids chenodeoxycholic acid and cholic acid. Plasma levels of 7α-hydroxy-4-cholesten-3-one were reduced, indicating repressed hepatic bile acid synthesis. There were minimal effects on indices of lipid, glucose, and energy metabolism. No serious adverse events were reported during GDCA administration in either capsule types, although 50% of participants showed mild increases in plasma levels of liver transaminases and 80% (regular capsules) and 50% (slow-release capsules) of participants experienced gastrointestinal adverse events. GDCA administration leads to elevated FGF19 levels and effectively inhibits primary bile acid synthesis, supporting therapy compliance and its effectiveness. However, effects on lipid, glucose, and energy metabolism were minimal, indicating that expanding the pool of this relatively hydrophobic bile acid does not impact energy metabolism in healthy subjects. See the paper by Emma Meessen!
October 2024: The aim of this work was to investigate the association between macronutrient intakes and continuous glucose monitoring (CGM) metrics in individuals with type 1 diabetes. In 470 individuals with type 1 diabetes of the GUTDM1 cohort (65% female, median age 40 [IQR 28-53] years, median diabetes duration 15 [IQR 6-29] years), we used logistic regression to establish associations between macronutrient intakes and the CGM metrics time in range (TIR, time spent between 3.9-10.0 mmol/l blood glucose, optimally set at ≥70%) and time below range (TBR, <3.9 mmol/l blood glucose, optimally set at <4%). ORs were expressed per 1 SD intake of nutrient and were adjusted for other macronutrient intakes, age, sex, socioeconomic status, BMI, duration of type 1 diabetes, pump use, insulin dose and alcohol intake. The median (IQR) TIR was 67 (51-80)% and TBR was 2 (1-4)%; the mean ± SD energy intake was 6879±2001 kJ, fat intake 75±31 g, carbohydrate intake 162±63 g, fibre intake 20±9 g and protein intake 70±24 g. A higher fibre intake and a lower carbohydrate intake were associated with higher odds of having a TIR≥70% (OR [95% CI] 1.64 [1.22, 2.24] and 0.67 [0.51, 0.87], respectively), whereas solely a higher carbohydrate intake was associated with TBR<4% (OR 1.34 [95% CI 1.02, 1.78]). A higher fibre intake is independently associated with a higher TIR. A higher carbohydrate intake is associated with less time spent in hypoglycaemia, a lower TIR and a higher time above range. These findings warrant confirmatory (interventional) investigations and may impact current nutritional guidelines for type 1 diabetes. Douwe the Wit wrote this paper.
June 2024: 3β-hydroxy-Δ5-C27-steroid-oxidoreductase (3β-HSD) deficiency is a bile acid synthesis disorder that leads to the absence of normal primary bile acids and the accumulation of abnormal bile acids. This results in cholestatic jaundice, fat-soluble vitamin deficiency, acholic or fatty stools and failure to thrive. Bile acid supplementation is used to treat 3β-HSD-deficiency and its symptoms. This report details the case of a 28-year-old woman diagnosed with 3β-HSD-deficiency, who was treated with glycine-conjugated deoxycholic acid (gDCA). gDCA treatment successfully restored normal bile acid levels, improved body weight by reducing fat malabsorption, and was well-tolerated with no observed liver problems or side effects. As a potent FXR ligand, gDCA might exert its action through FXR activation leading to bile acid synthesis regulation. Find the article here.
June 2024: In order to better understand which metabolic differences are related to insulin resistance in metabolic syndrome (MetSyn), we used hyperinsulinemic-euglycemic (HE) clamps in individuals with MetSyn and related peripheral insulin resistance to circulating biomarkers. In this cross-sectional study, HE-clamps were performed in treatment-naive men (n = 97) with MetSyn. Subjects were defined as insulin-resistant based on the rate of disappearance (Rd). Machine learning models and conventional statistics were used to identify biomarkers of insulin resistance. Findings were replicated in a cohort with n = 282 obese men and women with (n = 156) and without (n = 126) MetSyn. In addition to this, the relation between biomarkers and adipose tissue was assessed by nuclear magnetic resonance imaging. Peripheral insulin resistance is marked by changes in proteins related to inflammatory processes such as IL-1 and TNF-receptor and superfamily members. These proteins can distinguish between insulin-resistant and insulin-sensitive individuals (AUC = 0.72 ± 0.10) with MetSyn. These proteins were also associated with IFG, liver fat (rho 0.36, p = 1.79 × 10-9) and visceral adipose tissue (rho = 0.35, p = 6.80 × 10-9). Interestingly, these proteins had the strongest association in the MetSyn subgroup compared to individuals without MetSyn. MetSyn associated with insulin resistance is characterized by protein changes related to body fat content, insulin signaling and pro-inflammatory processes. These findings provide novel targets for intervention studies and should be the focus of future in vitro and in vivo studies. Another paper by Moritz Warmbrunn.
April 2024: Ageing changes the impact of nutrition, whereby inflammation has been suggested to play a role in age-related disabilities such as diabetes and cardiovascular disease. The aim of this study was to investigate differences in postprandial bile-acid response and its effect on energy metabolism between young and elderly people. Nine young, healthy men and nine elderly, healthy men underwent a liquid mixed-meal test. Postprandial bile-acid levels, insulin, glucose, GLP-1, C4, FGF19 and lipids were measured. Appetite, body composition, energy expenditure and gut microbiome were also measured. The elderly population showed lower glycine conjugated CDCA and UDCA levels and higher abundances of Ruminiclostridium, Marvinbryantia and Catenibacterium, but lower food intake, decreased fat free mass and increased cholesterol levels. Aging is associated with changes in postprandial bile-acid composition and microbiome, diminished hunger and changes in body composition and lipid levels. Further studies are needed to determine if these changes may contribute to malnutrition and sarcopenia in elderly. See the paper here in Pubmed!
March 2024: Gut microbiota have been linked to blood lipid levels and cardiovascular diseases (CVDs). The composition and abundance of gut microbiota trophic networks differ between ethnicities. We aim to evaluate the relationship between gut microbiotal trophic networks and CVD phenotypes. We included cross-sectional data from 3860 individuals without CVD history from 6 ethnicities living in the Amsterdam region participating in the prospective Healthy Life in Urban Setting (HELIUS) study. Genetic variants were genotyped, faecal gut microbiota were profiled, and blood and anthropometric parameters were measured. A machine learning approach was used to assess the relationship between CVD risk (Framingham score) and gut microbiota stratified by ethnicity. Potential causal relationships between gut microbiota composition and CVD were inferred by performing two-sample Mendelian randomization with hard CVD events from the Pan-UK Biobank and microbiome genome-wide association studies summary data from a subset of the HELIUS cohort (n = 4117). Microbial taxa identified to be associated with CVD by machine learning and Mendelian randomization were often ethnic-specific, but some concordance across ethnicities was found. The microbes Akkermansia muciniphila and Ruminococcaceae UCG-002 were protective against ischaemic heart disease in African-Surinamese and Moroccans, respectively. We identified a strong inverse association between blood lipids, CVD risk, and the combined abundance of the correlated microbes Christensenellaceae-Methanobrevibacter-Ruminococcaceae (CMR). The CMR cluster was also identified in two independent cohorts and the association with triglycerides was replicated. Certain gut microbes can have a potentially causal relationship with CVD events, with possible ethnic-specific effects. We identified a trophic network centred around Christensenellaceae, Methanobrevibacter, and various Ruminococcaceae, frequently lacking in South-Asian Surinamese, to be protective against CVD risk and associated with low triglyceride levels. Read the paper by Moritz Warmbrunn here!
November 2023: How the gut and thyroid interact! Graves’ disease (GD) and Graves’ orbitopathy (GO) result from ongoing stimulation of the TSH receptor due to autoantibodies acting as persistent agonists. Orbital pre-adipocytes and fibroblasts also express the TSH receptor, resulting in expanded retro-orbital tissue and causing exophthalmos and limited eye movement. Recent studies have shown that GD/GO patients have a disturbed gut microbiome composition, which has been associated with increased intestinal permeability. This study hypothesizes that enhanced intestinal permeability may aggravate orbital inflammation and, thus, increase myofibroblast differentiation and the degree of fibrosis. Two distinct cohorts of GO patients were studied, one of which was a unique cohort consisting of blood, fecal, and retro-orbital tissue samples. Intestinal permeability was assessed by measuring serum lipopolysaccharide-binding protein (LBP), zonulin, TLR5, and TLR9 ligands. The influx of macrophages and accumulation of T-cells and myofibroblast were quantified in orbital connective tissue. The NanoString immune-oncology RNA targets panel was used to determine the transcriptional profile of active fibrotic areas within orbital sections. GO patients displayed significantly higher LBP serum concentrations than healthy controls. Within the MicroGO cohort, patients with high serum LBP levels also showed higher levels of zonulin and TLR5 and TLR9 ligands in their circulation. The increased intestinal permeability was accompanied by augmented expression of genes marking immune cell infiltration and encoding key proteins for immune cell adhesion, antigen presentation, and cytokine signaling in the orbital tissue. Macrophage influx was positively linked to the extent of T cell influx and fibroblast activation within GO-affected orbital tissues. Moreover, serum LBP levels significantly correlated with the abundance of specific Gram-negative gut bacteria, linking the gut to local orbital inflammation. These results indicate that GO patients have enhanced intestinal permeability. The subsequent translocation of bacterial compounds to the systemic circulation may aggravate inflammatory processes within the orbital tissue and, as a consequence, augment the proportion of activated myofibroblasts, which actively secrete extracellular matrix leading to retro-orbital tissue expansion. These findings warrant further exploration to assess the correlation between specific inflammatory pathways in the orbital tissue and the gut microbiota composition and may pave the way for new microbiota-targeting therapies. See the paper by Aline Fenneman here!
October 2023: Soumia Majait write this paper on an inherited bile acid disorder. Cerebrotendinous xanthomatosis (CTX) is a rare inherited disease characterized by sterol 27-hydroxylase (CYP27A1) deficiency and, thus, a lack of bile acid synthesis with a marked accumulation of 7α-hydroxylated bile acid precursors. In addition to their renowned lipid-emulgating role, bile acids have been shown to stimulate secretion of the glucose-lowering and satiety-promoting gut hormone glucagon-like peptide 1 (GLP-1). In this paper, we examined postprandial bile acid, glucose, insulin, GLP-1 and fibroblast growth factor 19 (FGF19) plasma profiles in patients with CTX and matched healthy controls. Seven patients and seven age, gender and body mass index matched controls were included and subjected to a 4 h mixed meal test with regular blood sampling. CTX patients withdrew from chenodeoxycholic acid (CDCA) and statin therapy three weeks prior to the test. Postprandial levels of total bile acids were significantly lower in CTX patients and consisted of residual CDCA with low amounts of ursodeoxycholic acid (UDCA). The postprandial plasma glucose peak concentration occurred later in CTX patients compared to controls, and patients’ insulin levels remained elevated for a longer time. Postprandial GLP-1 levels were slightly higher in CTX subjects whereas postprandial FGF19 levels were lower in CTX subjects. This novel characterization of CTX patients reveals very low circulating bile acid levels and FGF19 levels, aberrant postprandial glucose and insulin profiles, and elevated postprandial GLP-1 responses. Read it here!

February 2023: This is about lifestyle. One of the most important topics in Western medicine. A healthy lifestyle is indispensable for the prevention of noncommunicable diseases. However, lifestyle medicine is hampered by time constraints and competing priorities of treating physicians. A dedicated lifestyle front office (LFO) in secondary/tertiary care may provide an important contribution to optimize patient-centred lifestyle care and connect to lifestyle initiatives from the community. The LOFIT study aims to gain insight into the (cost-)effectiveness of the LFO. Two parallel pragmatic randomized controlled trials will be conducted for (cardio)vascular disorders (i.e. (at risk of) (cardio)vascular disease, diabetes) and musculoskeletal disorders (i.e. osteoarthritis, hip or knee prosthesis). Patients from three outpatient clinics in the Netherlands will be invited to participate in the study. Inclusion criteria are body mass index (BMI) ≥25 (kg/m2) and/or smoking. Participants will be randomly allocated to either the intervention group or a usual care control group. In total, we aim to include 552 patients, 276 in each trial divided over both treatment arms. Patients allocated to the intervention group will participate in a face-to-face motivational interviewing (MI) coaching session with a so-called lifestyle broker. The patient will be supported and guided towards suitable community-based lifestyle initiatives. A network communication platform will be used to communicate between the lifestyle broker, patient, referred community-based lifestyle initiative and/or other relevant stakeholders (e.g. general practitioner). The primary outcome measure is the adapted Fuster-BEWAT, a composite health risk and lifestyle score consisting of resting systolic and diastolic blood pressure, objectively measured physical activity and sitting time, BMI, fruit and vegetable consumption and smoking behaviour. Secondary outcomes include cardiometabolic markers, anthropometrics, health behaviours, psychological factors, patient-reported outcome measures (PROMs), cost-effectiveness measures and a mixed-method process evaluation. Data collection will be conducted at baseline, 3, 6, 9 and 12 months follow-up. This study will gain insight into the (cost-)effectiveness of a novel care model in which patients under treatment in secondary or tertiary care are referred to community-based lifestyle initiatives to change their lifestyle. You find the protocol paper bij Marlinde van Dijk here.
July 2022: Again a project by Suzanne Meiring. The gut microbiota influences and interacts with the host metabolism through effects on nutrient metabolism and digestion. Duodenal Mucosal Resurfacing (DMR) is a novel endoscopic procedure involving duodenal mucosal ablation by the use of hydrothermal energy. DMR, when combined with a glucagon-like peptide-1 receptor agonist (GLP-1RA), resulted in discontinuation of exogenous insulin treatment in 69% of patients with insulin dependent type 2 diabetes mellitus (T2DM) in the INSPIRE study. These patients also experienced improved glycaemic control and metabolic health. We thus investigated if these clinical effects were associated with a change in gut microbiota alpha and beta diversity. Faecal samples from the 16 patients were obtained for Illumina shotgun sequencing at baseline and 3 months after DMR. We assessed alpha and beta diversity of the gut microbiota in these samples and analysed its correlations with changes in HbA1c, body weight, and liver MRI proton density fat fraction (PDFF). HbA1c correlated negatively with alpha diversity (p=0.011, rho: -0.62) whereas changes in PDFF correlated significantly with beta diversity (p=0.036, rho: 0.55) 3 months after initiation of the combined intervention. These correlations with metabolic parameters were observed despite finding no change in gut microbiota diversity at 3 months post DMR. The correlation between gut microbiota richness (alpha diversity) and HbA1c as well as the change in PDFF and changed microbiota composition (beta diversity) suggests that changed gut microbiota diversity is associated with metabolic improvements after DMR in combination with glucagon-like-peptide-1 receptor agonist in type 2 diabetes. Larger controlled studies are however needed to find causal links between DMR with GLP-1RA, the gut microbiota, and improvements in metabolic health.
February 2022: Back to diabetes: Duodenal mucosal resurfacing (DMR) is a new endoscopic ablation technique aimed at improving glycemia and metabolic control in patients with type 2 diabetes mellitus (T2DM). DMR appears to improve insulin resistance, which is the root cause of T2DM, but its mechanism of action is largely unknown. Bile acids function as intestinal signaling molecules in glucose and energy metabolism via the activation of farnesoid X receptor and secondary signaling [e.g., via fibroblast growth factor 19 (FGF19)], and are linked to metabolic health. We investigated the effect of DMR and glucagon-like peptide-1 (GLP-1) on postprandial bile acid responses in 16 patients with insulin-dependent T2DM, using mixed meal tests performed at the baseline and 6 mo after the DMR procedure. The combination treatment allowed discontinuation of insulin treatment in 11/16 (69%) of patients while improving glycemic and metabolic health. We found increased postprandial unconjugated bile acid responses (all P < 0.05), an overall increased secondary bile acid response (P = 0.036) and a higher 12α-hydroxylated:non-12α-hydroxylated ratio (P < 0.001). Total bile acid concentrations were unaffected by the intervention. Postprandial FGF19 and 7-α-hydroxy-4-cholesten-3-one (C4) concentrations decreased postintervention (both P < 0.01). Our study demonstrates that DMR with GLP-1 modulates the postprandial bile acid response. The alterations in postprandial bile acid responses may be the result of changes in the microbiome, ileal bile acid uptake and improved insulin sensitivity. Controlled studies are needed to elucidate the mechanism linking the combination treatment to metabolic health and bile acids. Suzanne Meiring wrote the paper!

January 2022: Background: Generally, food intake occurs in a three-meal per 24 h fashion with in-between meal snacking. As such, most humans spend more than ∼ 12-16 h per day in the postprandial state. It may be reasoned from an evolutionary point of view, that the human body is physiologically habituated to less frequent meals. Metabolic flexibility (i.e., reciprocal changes in carbohydrate and fatty acid oxidation) is a characteristic of metabolic health and is reduced by semi-continuous feeding. The effects of time-restricted feeding (TRF) on metabolic parameters and physical performance in humans are equivocal. Methods: To investigate the effect of TRF on metabolism and physical performance in free-living healthy lean individuals, we compared the effects of eucaloric feeding provided by a single meal (22/2) vs. three meals per day in a randomized crossover study. We included 13 participants of which 11 (5 males/6 females) completed the study: age 31.0 ± 1.7 years, BMI 24.0 ± 0.6 kg/m2 and fat mass (%) 24.0 ± 0.6 (mean ± SEM). Participants consumed all the calories needed for a stable weight in either three meals (breakfast, lunch and dinner) or one meal per day between 17:00 and 19:00 for 11 days per study period. Results: Eucaloric meal reduction to a single meal per day lowered total body mass (3 meals/day -0.5 ± 0.3 vs. 1 meal/day -1.4 ± 0.3 kg, p = 0.03), fat mass (3 meals/day -0.1 ± 0.2 vs. 1 meal/day -0.7 ± 0.2, p = 0.049) and increased exercise fatty acid oxidation (p < 0.001) without impairment of aerobic capacity or strength (p > 0.05). Furthermore, we found lower plasma glucose concentrations during the second half of the day during the one meal per day intervention (p < 0.05). Conclusion: A single meal per day in the evening lowers body weight and adapts metabolic flexibility during exercise via increased fat oxidation whereas physical performance was not affected. Emma Meessen did all the work, read it here!

June 2021: Covid19 affects our nutritional state. Patients with COVID-19 infection presents with a broad clinical spectrum of symptoms and complications. As a consequence nutritional requirements are not met, resulting in weight- and muscle loss, and malnutrition. The aim of the present study is to delineate nutritional complaints, the (course of the) nutritional status and risk of sarcopenia of COVID-19 patients, during hospitalisation and after discharge. In this prospective observational study in 407 hospital admitted COVID-19 patients in four university and peripheral hospitals, data were collected during dietetic consultations. Presence of nutrition related complaints (decreased appetite, loss of smell, changed taste, loss of taste, chewing and swallowing problems, nausea, vomiting, feeling of being full, stool frequency and consistency, gastric retention, need for help with food intake due to weakness and shortness of breath and nutritional status (weight loss, BMI, risk of sarcopenia with SARC-F ≥4 points) before, during hospital stay and after discharge were, where possible, collected. Included patients were most men (69%), median age of 64.8 ± 12.4 years, 60% were admitted to ICU at any time point during hospitalisation with a median LOS of 15 days and an in-hospital mortality rate of 21%. The most commonly reported complaints were: decreased appetite (58%), feeling of being full (49%) and shortness of breath (43%). One in three patients experienced changed taste, loss of taste and/or loss of smell. Prior to hospital admission, 67% of the patients was overweight (BMI >25 kg/m2), 35% of the patients was characterised as malnourished, mainly caused by considerable weight loss. Serious acute weight loss (>5 kg) was showed in 22% of the patents during the hospital stay; most of these patients (85%) were admitted to the ICU at any point in time. A high risk of sarcopenia (SARC-F ≥ 4 points) was scored in 73% of the patients during hospital admission. In conclusion, one in five hospital admitted COVID-19 patients suffered from serious acute weight loss and 73% had a high risk of sarcopenia. Moreover, almost all patients had one or more nutritional complaints. Of these complaints, decreased appetite, feeling of being full, shortness of breath and changed taste and loss of taste were the most predominant nutrition related complaints. These symptoms have serious repercussions on nutritional status. Although nutritional complaints persisted a long time after discharge, only a small group of patients received dietetic treatment after hospital discharge in recovery phase. Clinicians should consider the risks of acute malnutrition and sarcopenia in COVID-19 patients and investigate multidisciplinary treatment including dietetics during hospital stay and after discharge. See the paper by Nicolette Wierdsma here!

April 2021: Moritz Warbrunn did a nice clamp study: Metabolic syndrome (MetSyn) is an important risk factor for type 2 diabetes and cardiovascular diseases (CVD). This study aimed to find distinct plasma metabolite profiles between insulin-resistant and non-insulin resistant subjects with MetSyn and evaluate if MetSyn metabolite profiles are related to CVD risk and lipid fluxes. In a cross-sectional study, untargeted metabolomics of treatment-naive males with MetSyn (n = 132) were analyzed together with clinical parameters. In a subset of MetSyn participants, CVD risk was calculated using the Framingham score (n = 111), and lipolysis (n = 39) was measured by a two-step hyperinsulinemic euglycemic clamp using [1,1,2,3,3-2H5] glycerol to calculate lipolysis suppression rates. Peripheral insulin resistance was related to fatty acid metabolism and glycerolphosphorylcholine. Interestingly, although insulin resistance is considered to be a risk factor for CVD, we observed that there was little correspondence between metabolites associated with insulin resistance and metabolites associated with CVD risk. The latter mainly belonged to the androgenic steroid, fatty acid, phosphatidylethanolamine, and phophatidylcholine pathways. These data provide new insights into metabolic changes in mild MetSyn pathophysiology and MetSyn CVD risk related to lipid metabolism. Prospective studies may focus on the pathophysiological role of the here-identified biomarkers. Read it here!
 February 2021: In evolution, genes survived that could code for metabolic pathways, promoting long term survival during famines or fasting when suffering from trauma, disease or during physiological growth. This requires utilization of substrates, already present in some form in the body. Carbohydrate stores are limited and to survive long, their utilization is restricted to survival pathways, by inhibiting glucose oxidation and glycogen synthesis. This leads to insulin resistance and spares muscle protein, because being the main supplier of carbon for new glucose production. In these survival pathways, part of the glucose is degraded in glycolysis in peripheral (muscle) tissues to pyruvate and lactate (Warburg effect), which are partly reutilized for glucose formation in liver and kidney, completing the Cori-cycle. Another part of the glucose taken up by muscle contributes, together with muscle derived amino acids, to the production of substrates consisting of a complete amino acid mix but extra non-essential amino acids like glutamine, alanine, glycine and proline. These support cell proliferation, matrix deposition and redox regulation in tissues, specifically active in host response and during growth. In these tissues, also glucose is taken up delivering glycolytic intermediates, that branch off and act as building blocks and produce reducing equivalents. Lactate is also produced and released in the circulation, adding to the lactate released by muscle in the Cori-cycle and completing secondary glucose cycles. Increased fluxes through these cycles lead to modest hyperglycemia and hyperlactatemia in states of healthy growth and disease and are often misinterpreted as induced by hypoxia. You can find the paper here.
February 2021: In evolution, genes survived that could code for metabolic pathways, promoting long term survival during famines or fasting when suffering from trauma, disease or during physiological growth. This requires utilization of substrates, already present in some form in the body. Carbohydrate stores are limited and to survive long, their utilization is restricted to survival pathways, by inhibiting glucose oxidation and glycogen synthesis. This leads to insulin resistance and spares muscle protein, because being the main supplier of carbon for new glucose production. In these survival pathways, part of the glucose is degraded in glycolysis in peripheral (muscle) tissues to pyruvate and lactate (Warburg effect), which are partly reutilized for glucose formation in liver and kidney, completing the Cori-cycle. Another part of the glucose taken up by muscle contributes, together with muscle derived amino acids, to the production of substrates consisting of a complete amino acid mix but extra non-essential amino acids like glutamine, alanine, glycine and proline. These support cell proliferation, matrix deposition and redox regulation in tissues, specifically active in host response and during growth. In these tissues, also glucose is taken up delivering glycolytic intermediates, that branch off and act as building blocks and produce reducing equivalents. Lactate is also produced and released in the circulation, adding to the lactate released by muscle in the Cori-cycle and completing secondary glucose cycles. Increased fluxes through these cycles lead to modest hyperglycemia and hyperlactatemia in states of healthy growth and disease and are often misinterpreted as induced by hypoxia. You can find the paper here.
/s3/static.nrc.nl/spoetnik/files/2014/11/iStock_000037022924_Large.jpg) March 2021: Emma Meessen did it again! Great physiological study. To investigate the acute effects of intravenous vs enteral meal administration on circulating bile acid and gut hormone responses. In a randomized crossover design, we compared the effects of duodenal (via a nasoduodenal tube) vs parenteral (intravenous) administration over 180 min of identical mixed meals on circulating bile acid and gut hormone concentrations in eight healthy lean men. We analysed the bile acid and gut hormone responses in two periods: the intraprandial period from time point (T) 0 until T180 during meal administration and the postprandial period from T180 until T360, after discontinuation of meal administration. Intravenous meal administration decreased the intraprandial (AUC (μmol/L∗min) duodenal 1469 ± 284 vs intravenous 240 ± 39, p < 0.01) and postprandial bile acid response (985 ± 240 vs 223 ± 5, p < 0.05) and was accompanied by decreased gut hormone responses including glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, glucagon-like peptide 2 and fibroblast growth factor 19. Furthermore, intravenous meal administration elicited greater glucose concentrations, but similar insulin concentrations compared to enteral administration. Compared to enteral administration, parenteral nutrition results in lower postprandial bile acid and gut hormone responses in healthy lean men. This was accompanied by higher glucose concentrations in the face of similar insulin concentrations exposing a clear incretin effect of enteral mixed meal administration. The alterations in bile acid homeostasis were apparent after only one intravenous meal. The paper is published in Clinical Nutritition.
March 2021: Emma Meessen did it again! Great physiological study. To investigate the acute effects of intravenous vs enteral meal administration on circulating bile acid and gut hormone responses. In a randomized crossover design, we compared the effects of duodenal (via a nasoduodenal tube) vs parenteral (intravenous) administration over 180 min of identical mixed meals on circulating bile acid and gut hormone concentrations in eight healthy lean men. We analysed the bile acid and gut hormone responses in two periods: the intraprandial period from time point (T) 0 until T180 during meal administration and the postprandial period from T180 until T360, after discontinuation of meal administration. Intravenous meal administration decreased the intraprandial (AUC (μmol/L∗min) duodenal 1469 ± 284 vs intravenous 240 ± 39, p < 0.01) and postprandial bile acid response (985 ± 240 vs 223 ± 5, p < 0.05) and was accompanied by decreased gut hormone responses including glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, glucagon-like peptide 2 and fibroblast growth factor 19. Furthermore, intravenous meal administration elicited greater glucose concentrations, but similar insulin concentrations compared to enteral administration. Compared to enteral administration, parenteral nutrition results in lower postprandial bile acid and gut hormone responses in healthy lean men. This was accompanied by higher glucose concentrations in the face of similar insulin concentrations exposing a clear incretin effect of enteral mixed meal administration. The alterations in bile acid homeostasis were apparent after only one intravenous meal. The paper is published in Clinical Nutritition.
 December 2020: Duodenal mucosal resurfacing (DMR) is an endoscopic intervention in which the duodenal mucosa is ablated by hydrothermal energy. DMR improves glycemic control in patients with type 2 diabetes (T2D), most likely by altered duodenal signaling leading to insulin sensitization. We studied whether we could discontinue insulin use in T2D patients by combining DMR with glucagon-like peptide-1 receptor agonist (GLP-1RA) and lifestyle counseling. In this single-arm, single-center feasibility study in 16 insulin-treated patients with T2D (hemoglobin A1c [HbA1c] ≤8.0%, basal insulin <1 U/kg/day, C-peptide ≥.5 nmol/L), patients underwent a single DMR followed by a 2-week postprocedural diet, after which GLP-1RA (liraglutide) was introduced. Lifestyle counseling was provided per American Diabetes Association guidelines. The primary endpoint was percentage of patients without insulin with an HbA1c ≤7.5% (responders) at 6 months. Secondary endpoints were changes in multiple glycemic and metabolic parameters and percentage of responders at 12 and 18 months, respectively. All 16 patients underwent successful DMR without procedure-related serious adverse events. At 6 months, 69% of patients were off insulin therapy with an HbA1c ≤7.5%. At 12 and 18 months 56% and 53% remained off insulin, respectively. All patients significantly improved in the glycemic and metabolic parameters of homeostatic model assessment for insulin resistance, body mass index, weight, and liver fat fraction. In this feasibility study, the combination of a single DMR and GLP-1RA, supported by lifestyle counseling, eliminated the need for insulin therapy in most patients with T2D through 18 months postprocedure, with adequate beta-cell capacity, while improving glucose regulation and metabolic health in all patients. A randomized-sham controlled trial is currently initiated based on these results. Annieke van Baar wrote this paper that is published in Gastrointestinal Endoscopy.
December 2020: Duodenal mucosal resurfacing (DMR) is an endoscopic intervention in which the duodenal mucosa is ablated by hydrothermal energy. DMR improves glycemic control in patients with type 2 diabetes (T2D), most likely by altered duodenal signaling leading to insulin sensitization. We studied whether we could discontinue insulin use in T2D patients by combining DMR with glucagon-like peptide-1 receptor agonist (GLP-1RA) and lifestyle counseling. In this single-arm, single-center feasibility study in 16 insulin-treated patients with T2D (hemoglobin A1c [HbA1c] ≤8.0%, basal insulin <1 U/kg/day, C-peptide ≥.5 nmol/L), patients underwent a single DMR followed by a 2-week postprocedural diet, after which GLP-1RA (liraglutide) was introduced. Lifestyle counseling was provided per American Diabetes Association guidelines. The primary endpoint was percentage of patients without insulin with an HbA1c ≤7.5% (responders) at 6 months. Secondary endpoints were changes in multiple glycemic and metabolic parameters and percentage of responders at 12 and 18 months, respectively. All 16 patients underwent successful DMR without procedure-related serious adverse events. At 6 months, 69% of patients were off insulin therapy with an HbA1c ≤7.5%. At 12 and 18 months 56% and 53% remained off insulin, respectively. All patients significantly improved in the glycemic and metabolic parameters of homeostatic model assessment for insulin resistance, body mass index, weight, and liver fat fraction. In this feasibility study, the combination of a single DMR and GLP-1RA, supported by lifestyle counseling, eliminated the need for insulin therapy in most patients with T2D through 18 months postprocedure, with adequate beta-cell capacity, while improving glucose regulation and metabolic health in all patients. A randomized-sham controlled trial is currently initiated based on these results. Annieke van Baar wrote this paper that is published in Gastrointestinal Endoscopy.
September 2020: In recent years, the human gut microbiome has been found to influence a multitude of non-communicable diseases such as cardiovascular disease and metabolic syndrome, with its components type 2 diabetes mellitus and obesity. It is recognized to be mainly influenced by environmental factors, such as lifestyle, but also genetics may play a role. The interaction of gut microbiota and obesity has been widely studied, but in regard to non-alcoholic fatty liver disease (NAFLD) as a manifestation of obesity and insulin resistance, the causal role of the gut microbiome has not been fully established. The mechanisms by which the gut microbiome influences lipid accumulation, inflammatory responses, and occurrence of fibrosis in the liver are a topic of active research. In addition, the influence of exercise on gut microbiome composition is also being investigated. In clinical trials, exercise reduced hepatic steatosis independently of weight reduction. Other studies indicate that exercise may modulate the gut microbiome. This puts forward the question whether exercise could mediate its beneficial effects on NAFLD via changes in gut microbiome. Yet, the specific mechanisms underlying this potential connection are largely unknown. Thus, associative evidence from clinical trials, as well as mechanistic studies in vivo are called for to elucidate the relationship between exercise and the gut microbiome in NAFLD. Here, Veera Houttu reviews the current literature on exercise and the gut microbiome in NAFLD.
June 2020: The postprandial state is the period in which the largest metabolic, endocrine and inflammatory changes occur in normal, healthy, day-to-day living. Metabolic substrates will find their way to target organs and these movements are accompanied by profound endocrine changes and mild inflammation. The postprandial period has a powerful anabolic capacity, but at the same time it is also a risk factor for cardiovascular disease It should not be neglected that most human beings are in the postprandial state for the largest part of the day. Depending on the size of the meal or the used definition, the postprandial state will last for 4–5 h. Multiplied by the number of meals on an average day, one can easily reach 15–16 h of postprandial state. This means that more hours per day are spend with relative high plasma glucose and insulin levels which result in high carbohydrate oxidation rates and the storage of both carbohydrates and lipids in adipose tissue. In contrast, less hours per day spent in the postprandial state allow plasma glucose levels to drop, fatty acid oxidation rates to increase and adipose tissue stores to dwindle. The global continuous feeding pattern likely reduces the normal physiological organism’s capacity to switch between substrates. This is also called “metabolic flexibility” and is perceived as a measure of healthy metabolism. This editorial disucces the relevance of food intake sequence that modulates postprandial glycemia. It refers to the paper by Sun and coworkers in Clinical Nutrition.
Marc h 2020: Bile acids are multifaceted metabolic compounds that signal to cholesterol, glucose, and lipid homeostasis via receptors like the Farnesoid X Receptor (FXR) and transmembrane Takeda G protein-coupled receptor 5 (TGR5). The postprandial increase in plasma bile acid concentrations is therefore a potential metabolic signal. However, this postprandial response has a high interindividual variability. Such variability may affect bile acid receptor activation. In this study, we analyzed the inter- and intraindividual variability of fasting and postprandial bile acid concentrations during three identical meals on separate days in eight healthy lean male subjects using a statistical and mathematical approach. The postprandial bile acid responses exhibited large interindividual and intraindividual variability. The individual mathematical models, which represent the enterohepatic circulation of bile acids in each subject, suggest that interindividual variability results from quantitative and qualitative differences of distal active uptake, colon transit, and microbial bile acid transformation. Conversely, intraindividual variations in gallbladder kinetics can explain intraindividual differences in the postprandial responses. Emma Meessen conclude that there is considerable inter- and intraindividual variation in postprandial plasma bile acid levels. The presented personalized approach is a promising tool to identify unique characteristics of underlying physiological processes and can be applied to investigate bile acid metabolism in pathophysiological conditions. The paper is published in Physiological Reports (free access!!).
h 2020: Bile acids are multifaceted metabolic compounds that signal to cholesterol, glucose, and lipid homeostasis via receptors like the Farnesoid X Receptor (FXR) and transmembrane Takeda G protein-coupled receptor 5 (TGR5). The postprandial increase in plasma bile acid concentrations is therefore a potential metabolic signal. However, this postprandial response has a high interindividual variability. Such variability may affect bile acid receptor activation. In this study, we analyzed the inter- and intraindividual variability of fasting and postprandial bile acid concentrations during three identical meals on separate days in eight healthy lean male subjects using a statistical and mathematical approach. The postprandial bile acid responses exhibited large interindividual and intraindividual variability. The individual mathematical models, which represent the enterohepatic circulation of bile acids in each subject, suggest that interindividual variability results from quantitative and qualitative differences of distal active uptake, colon transit, and microbial bile acid transformation. Conversely, intraindividual variations in gallbladder kinetics can explain intraindividual differences in the postprandial responses. Emma Meessen conclude that there is considerable inter- and intraindividual variation in postprandial plasma bile acid levels. The presented personalized approach is a promising tool to identify unique characteristics of underlying physiological processes and can be applied to investigate bile acid metabolism in pathophysiological conditions. The paper is published in Physiological Reports (free access!!).
 February 2020: Insulin resistance develops prior to the onset of overt type 2 diabetes, making its early detection vital. Direct accurate evaluation is currently only possible with complex examinations like the stable isotope-based hyperinsulinemic euglycemic clamp (HIEC). Metabolomic profiling enables the detection of thousands of plasma metabolites, providing a tool to identify novel biomarkers in human obesity. Liquid chromatography mass spectrometry-based untargeted plasma metabolomics was applied in 60 participants with obesity with a large range of peripheral insulin sensitivity as determined via a two-step HIEC with stable isotopes [6,6-2H2]glucose and [1,1,2,3,3-2H5]glycerol. This additionally enabled measuring insulin-regulated lipolysis, which combined with metabolomics, to the knowledge of this research group, has not been reported on before. Several plasma metabolites were identified that significantly correlated with glucose and lipid fluxes, led by plasma (gamma-glutamyl)citrulline, followed by betaine, beta-cryptoxanthin, fructosyllysine, octanylcarnitine, sphingomyelin (d18:0/18:0, d19:0/17:0) and thyroxine. Subsequent machine learning analysis showed that a panel of these metabolites derived from a number of metabolic pathways may be used to predict insulin resistance, dominated by non-essential amino acid citrulline and its metabolite gamma-glutamylcitrulline. This approach revealed a number of plasma metabolites that correlated reasonably well with glycemic and lipolytic flux parameters, measured using gold standard techniques. These metabolites may be used to predict the rate of glucose disposal in humans with obesity to a similar extend as HOMA, thus providing potential novel biomarkers for insulin resistance. Annick Hartstra published this paper here!
February 2020: Insulin resistance develops prior to the onset of overt type 2 diabetes, making its early detection vital. Direct accurate evaluation is currently only possible with complex examinations like the stable isotope-based hyperinsulinemic euglycemic clamp (HIEC). Metabolomic profiling enables the detection of thousands of plasma metabolites, providing a tool to identify novel biomarkers in human obesity. Liquid chromatography mass spectrometry-based untargeted plasma metabolomics was applied in 60 participants with obesity with a large range of peripheral insulin sensitivity as determined via a two-step HIEC with stable isotopes [6,6-2H2]glucose and [1,1,2,3,3-2H5]glycerol. This additionally enabled measuring insulin-regulated lipolysis, which combined with metabolomics, to the knowledge of this research group, has not been reported on before. Several plasma metabolites were identified that significantly correlated with glucose and lipid fluxes, led by plasma (gamma-glutamyl)citrulline, followed by betaine, beta-cryptoxanthin, fructosyllysine, octanylcarnitine, sphingomyelin (d18:0/18:0, d19:0/17:0) and thyroxine. Subsequent machine learning analysis showed that a panel of these metabolites derived from a number of metabolic pathways may be used to predict insulin resistance, dominated by non-essential amino acid citrulline and its metabolite gamma-glutamylcitrulline. This approach revealed a number of plasma metabolites that correlated reasonably well with glycemic and lipolytic flux parameters, measured using gold standard techniques. These metabolites may be used to predict the rate of glucose disposal in humans with obesity to a similar extend as HOMA, thus providing potential novel biomarkers for insulin resistance. Annick Hartstra published this paper here!
 February 2020: Cardiometabolic diseases (CMD) are a group of interrelated disorders such as metabolic syndrome, type 2 diabetes mellitus and cardiovascular diseases (CVD). As the prevalence of these diseases increases globally, efficient new strategies are necessary to target CMD and modifiable risk factors. In the past decade, evidence has accumulated regarding the influence of gut microbiota (GM) on CMD, providing new targets for therapeutic interventions. This narrative review discusses the pathophysiologic link between CMD, GM, and potential microbiota-based targets against atherosclerosis and modifiable risk factors for atherosclerosis. Low-grade inflammation can be induced through GM and its derived metabolites. CMD are influenced by GM and microbiota-derived metabolites such as short-chain fatty acids (SCFA), secondary bile acids, trimethylamine N-oxide (TMAO), and the composition of GM can modulate host metabolism. All of the above can lead to promising therapeutic targets. Most data are derived from animal models or human association studies; therefore, more translational and interventional research in humans is necessary to validate these promising findings. Reproduced findings such as aberrant microbiota patterns or circulating biomarkers could be targeted depending on individual metabolic profiles, moving toward personalized medicine in CMD. Moritz Warmbrunn Wrote the paper which is published in Expert Review of Endocrinology & Metabolism.
February 2020: Cardiometabolic diseases (CMD) are a group of interrelated disorders such as metabolic syndrome, type 2 diabetes mellitus and cardiovascular diseases (CVD). As the prevalence of these diseases increases globally, efficient new strategies are necessary to target CMD and modifiable risk factors. In the past decade, evidence has accumulated regarding the influence of gut microbiota (GM) on CMD, providing new targets for therapeutic interventions. This narrative review discusses the pathophysiologic link between CMD, GM, and potential microbiota-based targets against atherosclerosis and modifiable risk factors for atherosclerosis. Low-grade inflammation can be induced through GM and its derived metabolites. CMD are influenced by GM and microbiota-derived metabolites such as short-chain fatty acids (SCFA), secondary bile acids, trimethylamine N-oxide (TMAO), and the composition of GM can modulate host metabolism. All of the above can lead to promising therapeutic targets. Most data are derived from animal models or human association studies; therefore, more translational and interventional research in humans is necessary to validate these promising findings. Reproduced findings such as aberrant microbiota patterns or circulating biomarkers could be targeted depending on individual metabolic profiles, moving toward personalized medicine in CMD. Moritz Warmbrunn Wrote the paper which is published in Expert Review of Endocrinology & Metabolism.
 December 2019: The importance of the postprandial state has been acknowledged since hyperglycemia and hyperlipidemia are linked with several chronic systemic low-grade inflammation conditions. Humans per day spend more than 16 hours in the postprandial state and the postprandial state is acknowledged as a complex interplay between nutrients, hormones and diet derived metabolites. The purpose of this review is to provide insight into the physiology of the postprandial inflammatory response, the role of different nutrients, the pro-inflammatory effects of metabolic endotoxemia and the anti-inflammatory effects of bile acids. Moreover, we discuss nutritional strategies that may be linked to the described pathways to modulate the inflammatory component of the postprandial response. Keep in mind that some degree of inflammation might be physiological! Emma Meessen wrote the paper and it is published in Nutrients.
December 2019: The importance of the postprandial state has been acknowledged since hyperglycemia and hyperlipidemia are linked with several chronic systemic low-grade inflammation conditions. Humans per day spend more than 16 hours in the postprandial state and the postprandial state is acknowledged as a complex interplay between nutrients, hormones and diet derived metabolites. The purpose of this review is to provide insight into the physiology of the postprandial inflammatory response, the role of different nutrients, the pro-inflammatory effects of metabolic endotoxemia and the anti-inflammatory effects of bile acids. Moreover, we discuss nutritional strategies that may be linked to the described pathways to modulate the inflammatory component of the postprandial response. Keep in mind that some degree of inflammation might be physiological! Emma Meessen wrote the paper and it is published in Nutrients.
November 2019: Forgot to add this one! Intake of a high-fat meal induces a systemic inflammatory response in the postprandial which is augmented in obese subjects. However, the underlying mechanisms of this response have not been fully elucidated. We aimed to assess the effect of gut microbiota modulation on postprandial inflammatory response in lean and obese subjects. Ten lean and ten obese subjects with metabolic syndrome received oral vancomycin 500 mg four times per day for 7 days. Oral high-fat meal tests (50 g fat/m2 body surface area) were performed before and after vancomycin intervention. Gut microbiota composition, leukocyte counts, plasma lipopolysaccharides (LPS), LPS-binding protein (LBP), IL-6 and MCP-1 concentrations and monocyte CCR2 and cytokine expression were determined before and after the high-fat meal. Oral vancomycin treatment resulted in profound changes in gut microbiota composition and significantly decreased bacterial diversity in both groups (phylogenetic diversity pre- versus post-intervention: lean, 56.9 ± 7.8 vs. 21.4 ± 6.6, P < 0.001; obese, 53.9 ± 7.8 vs. 21.0 ± 5.9, P < 0.001). After intervention, fasting plasma LPS significantly increased (lean, median [IQR] 0.81 [0.63-1.45] EU/mL vs. 2.23 [1.33-3.83] EU/mL, P = 0.017; obese, median [IQR] 0.76 [0.45-1.03] EU/mL vs. 1.44 [1.11-4.24], P = 0.014). However, postprandial increases in leukocytes and plasma LPS were unaffected by vancomycin in both groups. Moreover, we found no changes in plasma LBP, IL-6 and MCP-1 or in monocyte CCR2 expression. Despite major vancomycin-induced disruption of the gut microbiota and increased fasting plasma LPS, the postprandial inflammatory phenotype in lean and obese subjects was unaffected in this study. Guido Bakker published the paper in Physiological Reports and it is free!
one! Intake of a high-fat meal induces a systemic inflammatory response in the postprandial which is augmented in obese subjects. However, the underlying mechanisms of this response have not been fully elucidated. We aimed to assess the effect of gut microbiota modulation on postprandial inflammatory response in lean and obese subjects. Ten lean and ten obese subjects with metabolic syndrome received oral vancomycin 500 mg four times per day for 7 days. Oral high-fat meal tests (50 g fat/m2 body surface area) were performed before and after vancomycin intervention. Gut microbiota composition, leukocyte counts, plasma lipopolysaccharides (LPS), LPS-binding protein (LBP), IL-6 and MCP-1 concentrations and monocyte CCR2 and cytokine expression were determined before and after the high-fat meal. Oral vancomycin treatment resulted in profound changes in gut microbiota composition and significantly decreased bacterial diversity in both groups (phylogenetic diversity pre- versus post-intervention: lean, 56.9 ± 7.8 vs. 21.4 ± 6.6, P < 0.001; obese, 53.9 ± 7.8 vs. 21.0 ± 5.9, P < 0.001). After intervention, fasting plasma LPS significantly increased (lean, median [IQR] 0.81 [0.63-1.45] EU/mL vs. 2.23 [1.33-3.83] EU/mL, P = 0.017; obese, median [IQR] 0.76 [0.45-1.03] EU/mL vs. 1.44 [1.11-4.24], P = 0.014). However, postprandial increases in leukocytes and plasma LPS were unaffected by vancomycin in both groups. Moreover, we found no changes in plasma LBP, IL-6 and MCP-1 or in monocyte CCR2 expression. Despite major vancomycin-induced disruption of the gut microbiota and increased fasting plasma LPS, the postprandial inflammatory phenotype in lean and obese subjects was unaffected in this study. Guido Bakker published the paper in Physiological Reports and it is free!
 September 2019: Bile acids, glucagon-like peptide-1 (GLP-1), and fibroblast growth factor 19 (FGF19) play an important role in postprandial metabolism. In this study, we investigated the postprandial bile acid response in plasma and its relation to insulin, GLP-1, and FGF19. First, we investigated the postprandial response to 40-h fast. Then we administered glycine-conjugated deoxycholic acid (gDCA) with the meal. We performed two separate observational randomized crossover studies on healthy, lean men. In experiment 1: we tested 4-h mixed meal after an overnight fast and a 40-h fast. In experiment 2, we tested a 4-h mixed meal test with and without gDCA supplementation. Both studies measured postprandial glucose, insulin, bile acids, GLP-1, and FGF19. In experiment 1, 40 h of fasting induced insulin resistance and increased postprandial GLP-1 and FGF19 concentrations. After an overnight fast, we observed strong correlations between postprandial insulin and gDCA levels at specific time points. In experiment 2, administration of gDCA increased GLP-1 levels and lowered late postprandial glucose without effect on FGF19. Energy expenditure was not affected by gDCA administration. Unexpectedly, 40 h of fasting increased both GLP-1 and FGF19, where the former appeared bile acid independent and the latter bile acid dependent. Second, a single dose of gDCA increased postprandial GLP-1. Therefore, our data add complexity to the physiological regulation of the enterokines GLP-1 and FGF19 by bile acids. You find the paper here!
September 2019: Bile acids, glucagon-like peptide-1 (GLP-1), and fibroblast growth factor 19 (FGF19) play an important role in postprandial metabolism. In this study, we investigated the postprandial bile acid response in plasma and its relation to insulin, GLP-1, and FGF19. First, we investigated the postprandial response to 40-h fast. Then we administered glycine-conjugated deoxycholic acid (gDCA) with the meal. We performed two separate observational randomized crossover studies on healthy, lean men. In experiment 1: we tested 4-h mixed meal after an overnight fast and a 40-h fast. In experiment 2, we tested a 4-h mixed meal test with and without gDCA supplementation. Both studies measured postprandial glucose, insulin, bile acids, GLP-1, and FGF19. In experiment 1, 40 h of fasting induced insulin resistance and increased postprandial GLP-1 and FGF19 concentrations. After an overnight fast, we observed strong correlations between postprandial insulin and gDCA levels at specific time points. In experiment 2, administration of gDCA increased GLP-1 levels and lowered late postprandial glucose without effect on FGF19. Energy expenditure was not affected by gDCA administration. Unexpectedly, 40 h of fasting increased both GLP-1 and FGF19, where the former appeared bile acid independent and the latter bile acid dependent. Second, a single dose of gDCA increased postprandial GLP-1. Therefore, our data add complexity to the physiological regulation of the enterokines GLP-1 and FGF19 by bile acids. You find the paper here!
 January 2019: Very glad to have contributed to this clinical project! Background: Patients with chronic intestinal failure (CIF) often develop cholestatic liver injury which may lead to liver failure and need for organ transplantation. Objective: Aim of this study was to investigate whether citrulline (CIT) and the enterokine FGF19 are associated with chronic cholestasis and overall survival (OS) in adult CIF patients, and to develop a risk score to predict their survival. Methods: We studied 135 adult CIF patients on iv supplementation (>3 months). Association of plasma CIT and FGF19 with chronic cholestasis and OS were estimated by logistic and Cox regression models. A predictive risk score was developed and validated internally. Results: Patients with chronic cholestasis (17%) had a reduced 5-year survival (38% vs 62%). In multivariable analysis, low FGF19, low CIT and female gender were associated with chronic cholestasis. Patients with low CIT (29% vs 69%) or low FGF19 (54% vs 66%) had reduced 5-year survival. Risk factors identified in multivariable analysis of OS were low FGF19 (HR 3.4), low CIT (HR 3.3) and number of iv infusions per week (HR 1.4). These three predictors were incorporated in a risk model of survival termed Model for End-Stage IF (MESIF) (C-statistic 0.78). The 5-year survival rates for patients with a MESIF score ranging from 0-20 (n=47), 20-40 (n=75) and >40 (n=13) were 80%, 58% and 14%, respectively. Conclusions: CIT and FGF19 predict chronic cholestasis and OS in this cohort of adult CIF patients, and the derived MESIF score is associated with their survival. Pending external validation, the MESIF score may help to identify patients for closer clinical monitoring or earlier referral to intestinal transplantation centers. The paper is written by Kiran Koelfat from Maastricht and will be published in the American Journal of Clinical Nutrition!
January 2019: Very glad to have contributed to this clinical project! Background: Patients with chronic intestinal failure (CIF) often develop cholestatic liver injury which may lead to liver failure and need for organ transplantation. Objective: Aim of this study was to investigate whether citrulline (CIT) and the enterokine FGF19 are associated with chronic cholestasis and overall survival (OS) in adult CIF patients, and to develop a risk score to predict their survival. Methods: We studied 135 adult CIF patients on iv supplementation (>3 months). Association of plasma CIT and FGF19 with chronic cholestasis and OS were estimated by logistic and Cox regression models. A predictive risk score was developed and validated internally. Results: Patients with chronic cholestasis (17%) had a reduced 5-year survival (38% vs 62%). In multivariable analysis, low FGF19, low CIT and female gender were associated with chronic cholestasis. Patients with low CIT (29% vs 69%) or low FGF19 (54% vs 66%) had reduced 5-year survival. Risk factors identified in multivariable analysis of OS were low FGF19 (HR 3.4), low CIT (HR 3.3) and number of iv infusions per week (HR 1.4). These three predictors were incorporated in a risk model of survival termed Model for End-Stage IF (MESIF) (C-statistic 0.78). The 5-year survival rates for patients with a MESIF score ranging from 0-20 (n=47), 20-40 (n=75) and >40 (n=13) were 80%, 58% and 14%, respectively. Conclusions: CIT and FGF19 predict chronic cholestasis and OS in this cohort of adult CIF patients, and the derived MESIF score is associated with their survival. Pending external validation, the MESIF score may help to identify patients for closer clinical monitoring or earlier referral to intestinal transplantation centers. The paper is written by Kiran Koelfat from Maastricht and will be published in the American Journal of Clinical Nutrition!
December 2018: This is a very nice project using clinical data. The nuclear receptor PPARγ is the master regulator of adipocyte differentiation, distribution, and function. In addition, PPARγ induces terminal differentiation of several epithelial cell lineages, including colon epithelia. Loss-of-function mutations in PPARG result in familial partial lipodystrophy subtype 3 (FPDL3), a rare condition characterized by aberrant adipose tissue distribution and severe metabolic complications, including diabetes. Mutations in PPARG have also been reported in sporadic colorectal cancers, but the significance of these mutations is unclear. Studying these natural PPARG mutations provides valuable insights into structure-function relationships in the PPARγ protein. We functionally characterized a novel FPLD3-associated PPARγ L451P mutation in helix 9 of the ligand binding domain (LBD). Interestingly, substitution of the adjacent amino acid K450 was previously reported in a human colon carcinoma cell line. We performed a detailed side-by-side functional comparison of these two PPARγ mutants. PPARγ L451P shows multiple intermolecular defects, including impaired cofactor binding and reduced RXRα heterodimerisation and subsequent DNA binding, but not in DBD-LBD interdomain communication. The K450Q mutant displays none of these functional defects. Other colon cancer-associated PPARγ mutants displayed diverse phenotypes, ranging from complete loss of activity to wildtype activity. Amino acid changes in helix 9 can differently affect LBD integrity and function. In addition, FPLD3-associated PPARγ mutations consistently cause intra- and/or intermolecular defects; colon cancer-associated PPARγ mutations on the other hand may play a role in colon cancer onset and progression, but this is not due to their effects on the most well-studied functional characteristics of PPARγ. The paper is published in Molecular Metabolism.
 December 2018: Placement of the duodenal-jejunal bypass liner (DJBL) leads to rapid weight loss and restoration of insulin sensitivity in a similar fashion to bariatric surgery. Increased systemic bile acid levels are candidate effectors for these effects through postprandial activation of their receptors TGR5 and FXR. We aimed to quantify postprandial bile acid, GLP-1 and FGF19 responses and assess their temporal relation to the weight loss and metabolic and hormonal changes seen after DJBL placement. We performed mixed meal testing in 17 obese patients with type 2 diabetes mellitus (DM2) directly before, one week after and 6 months after DJBL placement. Both fasting and postprandial bile acid levels were unchanged at 1 week after implantation, and greatly increased 6 months after implantation. The increase consisted of unconjugated bile acid species. 3 hr-postprandial GLP-1 levels increased after 1 week and were sustained, whereas FGF19 levels and postprandial plasma courses were unaffected. DJBL placement leads to profound increases in unconjugated bile acid levels after 6 months, similar to the effects of bariatric surgery. The temporal dissociation between the changes in bile acids, GLP-1 and FGF19 and other gut hormone responses warrant caution about the beneficial role of bile acids after DJBL placement. This observational uncontrolled study emphasizes the need for future controlled studies. The paper is a collaboration between Amsterdam and Maastricht. It is published in Metabolism.
December 2018: Placement of the duodenal-jejunal bypass liner (DJBL) leads to rapid weight loss and restoration of insulin sensitivity in a similar fashion to bariatric surgery. Increased systemic bile acid levels are candidate effectors for these effects through postprandial activation of their receptors TGR5 and FXR. We aimed to quantify postprandial bile acid, GLP-1 and FGF19 responses and assess their temporal relation to the weight loss and metabolic and hormonal changes seen after DJBL placement. We performed mixed meal testing in 17 obese patients with type 2 diabetes mellitus (DM2) directly before, one week after and 6 months after DJBL placement. Both fasting and postprandial bile acid levels were unchanged at 1 week after implantation, and greatly increased 6 months after implantation. The increase consisted of unconjugated bile acid species. 3 hr-postprandial GLP-1 levels increased after 1 week and were sustained, whereas FGF19 levels and postprandial plasma courses were unaffected. DJBL placement leads to profound increases in unconjugated bile acid levels after 6 months, similar to the effects of bariatric surgery. The temporal dissociation between the changes in bile acids, GLP-1 and FGF19 and other gut hormone responses warrant caution about the beneficial role of bile acids after DJBL placement. This observational uncontrolled study emphasizes the need for future controlled studies. The paper is a collaboration between Amsterdam and Maastricht. It is published in Metabolism.
 June 2018: Bile acids are terribly complicated and fulfill a variety of metabolic functions including regulation of glucose and lipid metabolism. Since changes of bile acid metabolism accompany obesity, Type 2 Diabetes Mellitus and bariatric surgery, there is great interest in their role in metabolic health. Here, we developed a mathematical model of systemic bile acid metabolism, and subsequently performed in silico analyses to gain quantitative insight into the factors determining plasma bile acid measurements. Intestinal transit was found to have a surprisingly central role in plasma bile acid appearance, as was evidenced by both the necessity of detailed intestinal transit functions for a physiological description of bile acid metabolism as well as the importance of the intestinal transit parameters in determining plasma measurements. The central role of intestinal transit is further highlighted by the dependency of the early phase of the dynamic response of plasma bile acids after a meal to intestinal propulsion. Fianne Sips shows this in Frontiers in Physiology which is open access!
June 2018: Bile acids are terribly complicated and fulfill a variety of metabolic functions including regulation of glucose and lipid metabolism. Since changes of bile acid metabolism accompany obesity, Type 2 Diabetes Mellitus and bariatric surgery, there is great interest in their role in metabolic health. Here, we developed a mathematical model of systemic bile acid metabolism, and subsequently performed in silico analyses to gain quantitative insight into the factors determining plasma bile acid measurements. Intestinal transit was found to have a surprisingly central role in plasma bile acid appearance, as was evidenced by both the necessity of detailed intestinal transit functions for a physiological description of bile acid metabolism as well as the importance of the intestinal transit parameters in determining plasma measurements. The central role of intestinal transit is further highlighted by the dependency of the early phase of the dynamic response of plasma bile acids after a meal to intestinal propulsion. Fianne Sips shows this in Frontiers in Physiology which is open access!

May 2018: The ability to efficiently adapt metabolism by substrate sensing, trafficking, storage and utilization, dependent on availability and requirement is known as metabolic flexibility. In this review, we discuss the breadth and depth of metabolic flexibility and its impact on health and disease. Metabolic flexibility is essential to maintain energy homeostasis in times of either caloric excess or caloric restriction, and in times of either low or high energy demand, such as during exercise. The liver, adipose tissue and muscle govern systemic metabolic flexibility and manage nutrient sensing, uptake, transport, storage and expenditure by communication via endocrine cues. At a molecular level, metabolic flexibility relies on the configuration of metabolic pathways which is regulated by key metabolic enzymes and transcription factors, many of which interact closely with the mitochondria. Disrupted metabolic flexibility, or metabolic inflexibility, however, is associated with many pathological conditions including metabolic syndrome, type 2 diabetes mellitus, and cancer. Multiple factors like dietary composition and feeding frequency, exercise training, and use of pharmacological compounds influence metabolic flexibility and will be discussed here. Lastly, we outline important advances in metabolic flexibility research and discuss medical horizons and translational aspects. The paper is published in Endocrine Reviews.
 February 2018: Anniek van Baar works on an exciting project that targets the duodenum for therapy in type 2 diabetes mellitus. Indeed, the Duodenum harbors a Broad Untapped Therapeutic Potential. The gastroenterologist, when performing an esophagogastroduodenoscopy, is the only medical care provider with easy access to the duodenum. This simple fact is pivotal in this article that discusses why the duodenum has become such an important anatomic region of interest.Recent insights have revealed the critical physiologic and pathophysiologic role of the small bowel in metabolic homeostasis and its potential role as a driver of obesity, insulin resistance, and subsequent type 2diabetes mellitus (T2DM). Although the other parts of the small bowel cannot be ignored when describing the potential mechanisms involved in the development of metabolic diseases and T2DM, the excellent endoscopic accessibility of the duodenum makes it a prime target for disease-modifying intervention. The paper is published in Gastroenterology.
February 2018: Anniek van Baar works on an exciting project that targets the duodenum for therapy in type 2 diabetes mellitus. Indeed, the Duodenum harbors a Broad Untapped Therapeutic Potential. The gastroenterologist, when performing an esophagogastroduodenoscopy, is the only medical care provider with easy access to the duodenum. This simple fact is pivotal in this article that discusses why the duodenum has become such an important anatomic region of interest.Recent insights have revealed the critical physiologic and pathophysiologic role of the small bowel in metabolic homeostasis and its potential role as a driver of obesity, insulin resistance, and subsequent type 2diabetes mellitus (T2DM). Although the other parts of the small bowel cannot be ignored when describing the potential mechanisms involved in the development of metabolic diseases and T2DM, the excellent endoscopic accessibility of the duodenum makes it a prime target for disease-modifying intervention. The paper is published in Gastroenterology.
December 2017: Its brain time again in Hannah Egginks paper that got a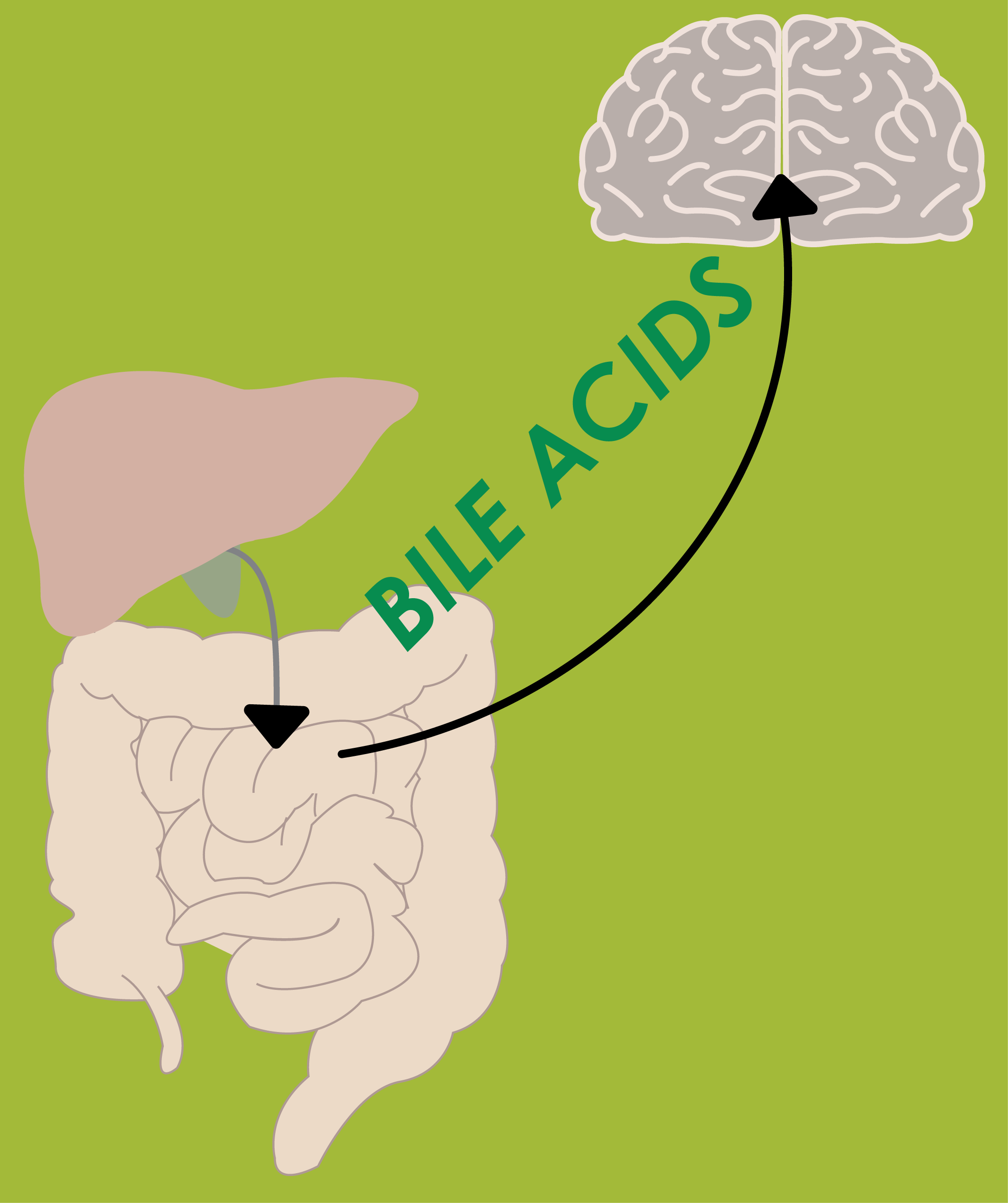 ccepted today! Bile acids can function in the postprandial state as circulating signaling molecules in the regulation of glucose and lipid metabolism via the transmembrane receptor TGR5 and nuclear receptor FXR. Both receptors are present in the central nervous system, but their function in the brain is unclear. Therefore, Hannah investigated the effects of intracerebroventricular (icv) administration of taurolithocholate (tLCA), a strong TGR5 agonist, and GW4064, a synthetic FXR agonist, on energy metabolism. She determined the effects of chronic icv infusion of tLCA, GW4064, or vehicle on energy expenditure, body weight and composition as well as tissue specific fatty acid uptake in mice equipped with osmotic minipumps. Icv administration of tLCA (final concentration in cerebrospinal fluid: 1μM) increased fat oxidation (tLCA group: 0.083±0.006 vs control group: 0.036±0.023 kcal/h, F=5.46, p=0.04) and decreased fat mass (after 9 days of tLCA infusion: 1.35±0.13 vs controls: 1.96±0.23 g, p=0.03). These changes were associated with enhanced uptake of triglyceride-derived fatty acids by brown adipose tissue and with browning of subcutaneous white adipose tissue. Icv administration of GW4064 (final concentration in cerebrospinal fluid: 10μM) did not affect energy metabolism, body composition nor bile acid levels, negating a role of FXR in the central nervous system in metabolic control. Bile acids such as tLCA may exert metabolic effects on fat metabolism via the brain. The paper is accepted in the Journal of Endocrinology!
ccepted today! Bile acids can function in the postprandial state as circulating signaling molecules in the regulation of glucose and lipid metabolism via the transmembrane receptor TGR5 and nuclear receptor FXR. Both receptors are present in the central nervous system, but their function in the brain is unclear. Therefore, Hannah investigated the effects of intracerebroventricular (icv) administration of taurolithocholate (tLCA), a strong TGR5 agonist, and GW4064, a synthetic FXR agonist, on energy metabolism. She determined the effects of chronic icv infusion of tLCA, GW4064, or vehicle on energy expenditure, body weight and composition as well as tissue specific fatty acid uptake in mice equipped with osmotic minipumps. Icv administration of tLCA (final concentration in cerebrospinal fluid: 1μM) increased fat oxidation (tLCA group: 0.083±0.006 vs control group: 0.036±0.023 kcal/h, F=5.46, p=0.04) and decreased fat mass (after 9 days of tLCA infusion: 1.35±0.13 vs controls: 1.96±0.23 g, p=0.03). These changes were associated with enhanced uptake of triglyceride-derived fatty acids by brown adipose tissue and with browning of subcutaneous white adipose tissue. Icv administration of GW4064 (final concentration in cerebrospinal fluid: 10μM) did not affect energy metabolism, body composition nor bile acid levels, negating a role of FXR in the central nervous system in metabolic control. Bile acids such as tLCA may exert metabolic effects on fat metabolism via the brain. The paper is accepted in the Journal of Endocrinology!
N ovember 2017: Bile acids are best known as detergents involved in the digestion of lipids. In addition, new data in the last decade have shown that bile acids also function as gut hormones capable of influencing metabolic processes via receptors such as FXR (farnesoid X receptor) and TGR5 (Takeda G protein-coupled receptor 5). These effects of bile acids are not restricted to the gastrointestinal tract, but can affect different tissues throughout the organism. It is still unclear whether these effects also involve signaling of bile acids to the central nervous system (CNS). Bile acid signaling to the CNS encompasses both direct and indirect pathways. Bile acids can act directly in the brain via central FXR and TGR5 signaling. In addition, there are two indirect pathways that involve intermediate agents released upon interaction with bile acids receptors in the gut. Activation of intestinal FXR and TGR5 receptors can result in the release of fibroblast growth factor 19 (FGF19) and glucagon-like peptide 1 (GLP-1), both capable of signaling to the CNS. We conclude that when plasma bile acids levels are high all three pathways may contribute in signal transmission to the CNS. However, under normal physiological circumstances, the indirect pathway involving GLP-1 may evoke the most substantial effect in the brain. Kim Mertens and Hannah Eggink tell you all about in our review paper that is published and freely accessible in Frontiers in Neuroscience!
ovember 2017: Bile acids are best known as detergents involved in the digestion of lipids. In addition, new data in the last decade have shown that bile acids also function as gut hormones capable of influencing metabolic processes via receptors such as FXR (farnesoid X receptor) and TGR5 (Takeda G protein-coupled receptor 5). These effects of bile acids are not restricted to the gastrointestinal tract, but can affect different tissues throughout the organism. It is still unclear whether these effects also involve signaling of bile acids to the central nervous system (CNS). Bile acid signaling to the CNS encompasses both direct and indirect pathways. Bile acids can act directly in the brain via central FXR and TGR5 signaling. In addition, there are two indirect pathways that involve intermediate agents released upon interaction with bile acids receptors in the gut. Activation of intestinal FXR and TGR5 receptors can result in the release of fibroblast growth factor 19 (FGF19) and glucagon-like peptide 1 (GLP-1), both capable of signaling to the CNS. We conclude that when plasma bile acids levels are high all three pathways may contribute in signal transmission to the CNS. However, under normal physiological circumstances, the indirect pathway involving GLP-1 may evoke the most substantial effect in the brain. Kim Mertens and Hannah Eggink tell you all about in our review paper that is published and freely accessible in Frontiers in Neuroscience!
September 2017: Induction of non-shivering thermogenesis can be used to influence energy balance to prevent or even treat obesity. The pungent component of mustard (allyl-isothiocyanate, AITC), activates the extreme cold receptor TRPA1 and may thus induce energy expenditure. During our postdoc in the TVP lab of Professor Toni Vidal-Puig, Mirjam Langeveld, Chong Yew Tan, Sam Virtue and I evaluated the potential of mustard AITC to induce thermogenesis (primary outcome) and alter body temperature, cold and hunger sensations, plasma metabolic parameters and energy intake (secondary outcomes). Energy expenditure in mice was measured after subcutaneous injection with vehicle, 1mg/kg noradrenaline or 5 mg/kg AITC. In the human study, with a crossover design, 10 healthy subjects were studied under temperature controlled conditions, after an overnight fast. After the ingestion of capsulated mustard (10 grams) or unpackaged mustard (10 grams) or capsulated placebo mixture, measurements of energy expenditure, substrate oxidation, core temperature, cold and hunger scores and plasma parameters were repeated every 30 minutes during 150 minutes. Subjects were randomised for the placebo and capsulated mustard intervention, nine out of ten subjects received the unpackaged mustard as final intervention since this could not be blinded. After the experiments were performed, energy intake was measured in a test meal, using the universal eating monitor. In mice AITC administration induced a 32% increase in energy expenditure compared to placebo (17.5±4.9 vs 12.5±1.2 J/min/mouse, p=0.03). Of the11 randomised participants one was excluded because of intercurrent illness and one did not return for the third visit (unpackaged mustard). Energy expenditure did not increase after capsulated or unpackaged mustard ingestion compared to placebo. No differences in substrate oxidation, core temperature, cold and hunger scores or plasma parameters were found, nor was the energy intake at the end of the experiment different between the three conditions. The highest tolerable dose of mustard we were able to use did not elicit a significant thermogenic response in humans. The paper will be published in the American Journal of Clinical Nutrition.
prevent or even treat obesity. The pungent component of mustard (allyl-isothiocyanate, AITC), activates the extreme cold receptor TRPA1 and may thus induce energy expenditure. During our postdoc in the TVP lab of Professor Toni Vidal-Puig, Mirjam Langeveld, Chong Yew Tan, Sam Virtue and I evaluated the potential of mustard AITC to induce thermogenesis (primary outcome) and alter body temperature, cold and hunger sensations, plasma metabolic parameters and energy intake (secondary outcomes). Energy expenditure in mice was measured after subcutaneous injection with vehicle, 1mg/kg noradrenaline or 5 mg/kg AITC. In the human study, with a crossover design, 10 healthy subjects were studied under temperature controlled conditions, after an overnight fast. After the ingestion of capsulated mustard (10 grams) or unpackaged mustard (10 grams) or capsulated placebo mixture, measurements of energy expenditure, substrate oxidation, core temperature, cold and hunger scores and plasma parameters were repeated every 30 minutes during 150 minutes. Subjects were randomised for the placebo and capsulated mustard intervention, nine out of ten subjects received the unpackaged mustard as final intervention since this could not be blinded. After the experiments were performed, energy intake was measured in a test meal, using the universal eating monitor. In mice AITC administration induced a 32% increase in energy expenditure compared to placebo (17.5±4.9 vs 12.5±1.2 J/min/mouse, p=0.03). Of the11 randomised participants one was excluded because of intercurrent illness and one did not return for the third visit (unpackaged mustard). Energy expenditure did not increase after capsulated or unpackaged mustard ingestion compared to placebo. No differences in substrate oxidation, core temperature, cold and hunger scores or plasma parameters were found, nor was the energy intake at the end of the experiment different between the three conditions. The highest tolerable dose of mustard we were able to use did not elicit a significant thermogenic response in humans. The paper will be published in the American Journal of Clinical Nutrition.
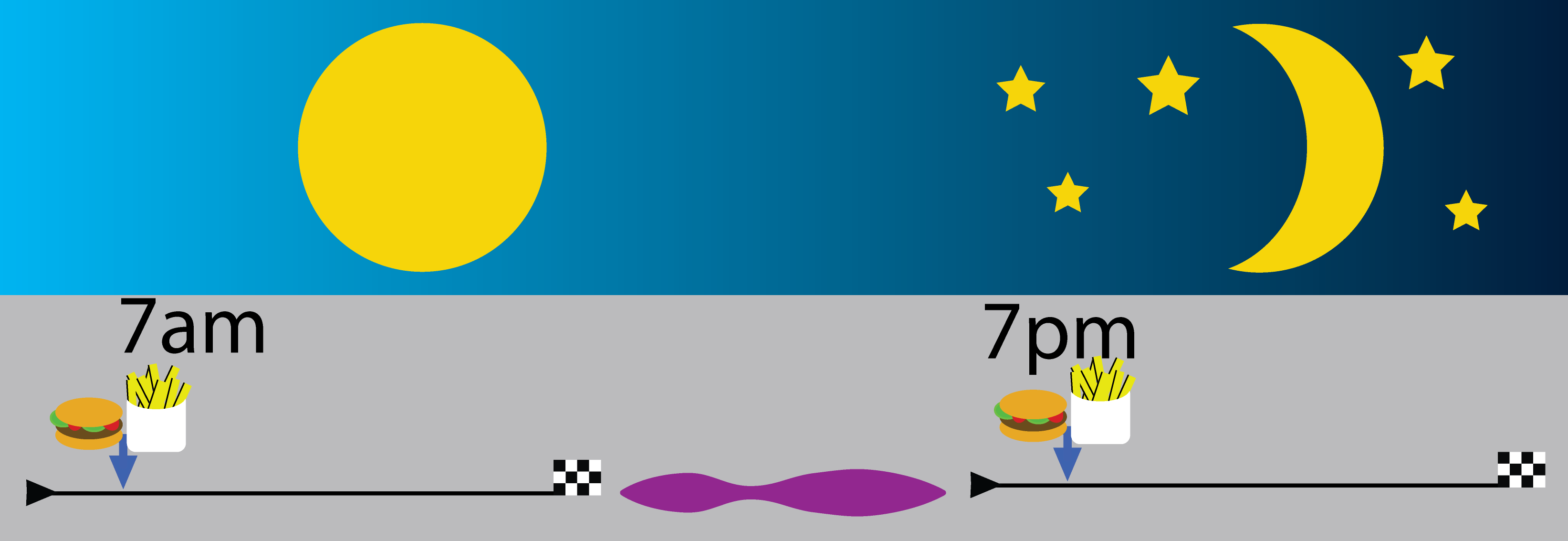 July 2017: Desynchronization between the master clock in the brain, which is entrained by (day) light, and peripheral organ clocks, which are mainly entrained by food intake, may have negative effects on energy metabolism. Bile acid metabolism follows a clear day/night rhythm. Hannah Eggink investigated whether in rats on a normal chow diet the daily rhythm of plasma bile acids and hepatic expression of bile acid metabolic genes is controlled by the light/dark cycle or the feeding/fasting rhythm. In addition, we investigated the effects of high caloric diets and time restricted feeding on daily rhythms of plasma bile acids and hepatic genes involved in bile acid synthesis. In experiment 1 male Wistar rats were fed according to 3 different feeding paradigms: food was available ad libitum for 24 h (ad lib) or time restricted for 10 h during the dark period (dark fed) or 10 h during the light period (light fed). To allow further metabolic phenotyping, we manipulated dietary macronutrient intake by providing rats with a chow diet, a free choice high-fat-high-sugar (HFHS) diet or a free choice high-fat (HF) diet. In experiment 2 rats were fed a normal chow diet, but food was either available in a 6-meals-a-day scheme (6M) or ad lib. During both experiments, we measured plasma bile acid levels and hepatic mRNA expression of genes involved in bile acid metabolism at 8 different time points during 24 h. Time restricted feeding enhanced the daily rhythm in plasma bile acid concentrations. Plasma bile acid concentrations are highest during fasting and dropped during the period of food intake with all diets. A high fat containing diet changed bile acid pool composition, but not the daily rhythmicity of plasma bile acid levels. Daily rhythms of hepatic Cyp7a1 and Cyp8b1 mRNA expression followed the hepatic molecular clock, whereas for Shp expression food intake was leading. Combining a high fat diet with feeding in the light/inactive period annulled CYp7a1 and Cyp8b1 gene expression rhythms, whilst keeping that of Shp intact. In conclusion, plasma bile acids and key genes in bile acid biosynthesis are entrained by food intake as well as the hepatic molecular clock. Eating during the inactivity period induced changes in the plasma bile acid pool composition similar to those induced by high fat feeding. The paper is published in Chronobiology International.
July 2017: Desynchronization between the master clock in the brain, which is entrained by (day) light, and peripheral organ clocks, which are mainly entrained by food intake, may have negative effects on energy metabolism. Bile acid metabolism follows a clear day/night rhythm. Hannah Eggink investigated whether in rats on a normal chow diet the daily rhythm of plasma bile acids and hepatic expression of bile acid metabolic genes is controlled by the light/dark cycle or the feeding/fasting rhythm. In addition, we investigated the effects of high caloric diets and time restricted feeding on daily rhythms of plasma bile acids and hepatic genes involved in bile acid synthesis. In experiment 1 male Wistar rats were fed according to 3 different feeding paradigms: food was available ad libitum for 24 h (ad lib) or time restricted for 10 h during the dark period (dark fed) or 10 h during the light period (light fed). To allow further metabolic phenotyping, we manipulated dietary macronutrient intake by providing rats with a chow diet, a free choice high-fat-high-sugar (HFHS) diet or a free choice high-fat (HF) diet. In experiment 2 rats were fed a normal chow diet, but food was either available in a 6-meals-a-day scheme (6M) or ad lib. During both experiments, we measured plasma bile acid levels and hepatic mRNA expression of genes involved in bile acid metabolism at 8 different time points during 24 h. Time restricted feeding enhanced the daily rhythm in plasma bile acid concentrations. Plasma bile acid concentrations are highest during fasting and dropped during the period of food intake with all diets. A high fat containing diet changed bile acid pool composition, but not the daily rhythmicity of plasma bile acid levels. Daily rhythms of hepatic Cyp7a1 and Cyp8b1 mRNA expression followed the hepatic molecular clock, whereas for Shp expression food intake was leading. Combining a high fat diet with feeding in the light/inactive period annulled CYp7a1 and Cyp8b1 gene expression rhythms, whilst keeping that of Shp intact. In conclusion, plasma bile acids and key genes in bile acid biosynthesis are entrained by food intake as well as the hepatic molecular clock. Eating during the inactivity period induced changes in the plasma bile acid pool composition similar to those induced by high fat feeding. The paper is published in Chronobiology International.
June 2017: Bile acids (BAs) play a key role in  lipid uptake and metabolic signalling in different organs including gut, liver, muscle and brown adipose tissue. Portal and peripheral plasma BA concentrations increase after a meal. However, the exact kinetics of postprandial BA metabolism have never been described in great detail. Hannah Eggink used a conscious porcine model to investigate postprandial plasma concentrations and transorgan fluxes of BAs, glucose and insulin using the para-aminohippuric acid dilution method. Eleven pigs with intravascular catheters received a standard mixed-meal while blood was sampled from different veins such as the portal vein, abdominal aorta and hepatic vein. To translate the data to humans, fasted venous and portal blood was sampled from non-diabetic obese patients during gastric by-pass surgery. The majority of the plasma bile acid pool and postprandial response consisted of glycine-conjugated forms of primary bile acids. Conjugated bile acids were more efficiently cleared by the liver than unconjugated forms. The timing and size of the postprandial response showed large interindividual variability for bile acids compared to glucose and insulin. The liver selectively extracts most BAs and BAs with highest affinity for the most important metabolic BA receptor, TGR5, are typically low in both porcine and human peripheral circulation. Our findings raise questions about the magnitude of a peripheral TGR5 signal and its ultimate clinical application. The paper is published in Clinical Nutrition.
lipid uptake and metabolic signalling in different organs including gut, liver, muscle and brown adipose tissue. Portal and peripheral plasma BA concentrations increase after a meal. However, the exact kinetics of postprandial BA metabolism have never been described in great detail. Hannah Eggink used a conscious porcine model to investigate postprandial plasma concentrations and transorgan fluxes of BAs, glucose and insulin using the para-aminohippuric acid dilution method. Eleven pigs with intravascular catheters received a standard mixed-meal while blood was sampled from different veins such as the portal vein, abdominal aorta and hepatic vein. To translate the data to humans, fasted venous and portal blood was sampled from non-diabetic obese patients during gastric by-pass surgery. The majority of the plasma bile acid pool and postprandial response consisted of glycine-conjugated forms of primary bile acids. Conjugated bile acids were more efficiently cleared by the liver than unconjugated forms. The timing and size of the postprandial response showed large interindividual variability for bile acids compared to glucose and insulin. The liver selectively extracts most BAs and BAs with highest affinity for the most important metabolic BA receptor, TGR5, are typically low in both porcine and human peripheral circulation. Our findings raise questions about the magnitude of a peripheral TGR5 signal and its ultimate clinical application. The paper is published in Clinical Nutrition.
 May 2017: Insulin resistance after surgery hampers recovery. Oxidative stress is shown to be involved in the occurrence of postoperative insulin resistance. Preoperative carbohydrate-rich oral nutrition supplements reduce but do not prevent insulin resistance. The aim of the study of Mireille van Stijn was to investigate the effect of a carbohydrate-, glutamine-, and antioxidant-enriched preoperative oral nutrition supplement on postoperative insulin resistance. A double-blind randomized controlled pilot study in 18 patients with rectal cancer, who received either the supplement (S) or the placebo (P) 15, 11, and 4 hours preoperatively, was conducted. Insulin sensitivity was studied prior to surgery and on the first postoperative day using a hyperinsulinemic euglycemic 2-step clamp. Hepatic insulin sensitivity (insulin-mediated suppression of glucose production) decreased significantly after surgery in both groups, with no differences between the groups. Peripheral insulin sensitivity (glucose rate of disappearance, Rd) was significantly decreased after surgery in both groups (S: 37.2 [19.1-50.9] vs 20.6 [13.9-27.9]; P: 23.8 [15.7-35.5] vs 15.3 [12.6-19.1] µmol/kg·min) but less pronounced in the supplemented group ( P = .04). The percentage decrease in glucose Rd did not differ between the groups. Adipose tissue insulin sensitivity (insulin-mediated suppression of plasma free fatty acids) decreased to the same extent after surgery in both groups. Rectal cancer surgery induced profound insulin resistance, affecting glucose and fatty acid metabolism. The preoperative nutrition supplement somewhat attenuated but did not prevent postoperative peripheral insulin resistance. The paper is published in the Journal of Parenteral and Enteral Nutrition.
May 2017: Insulin resistance after surgery hampers recovery. Oxidative stress is shown to be involved in the occurrence of postoperative insulin resistance. Preoperative carbohydrate-rich oral nutrition supplements reduce but do not prevent insulin resistance. The aim of the study of Mireille van Stijn was to investigate the effect of a carbohydrate-, glutamine-, and antioxidant-enriched preoperative oral nutrition supplement on postoperative insulin resistance. A double-blind randomized controlled pilot study in 18 patients with rectal cancer, who received either the supplement (S) or the placebo (P) 15, 11, and 4 hours preoperatively, was conducted. Insulin sensitivity was studied prior to surgery and on the first postoperative day using a hyperinsulinemic euglycemic 2-step clamp. Hepatic insulin sensitivity (insulin-mediated suppression of glucose production) decreased significantly after surgery in both groups, with no differences between the groups. Peripheral insulin sensitivity (glucose rate of disappearance, Rd) was significantly decreased after surgery in both groups (S: 37.2 [19.1-50.9] vs 20.6 [13.9-27.9]; P: 23.8 [15.7-35.5] vs 15.3 [12.6-19.1] µmol/kg·min) but less pronounced in the supplemented group ( P = .04). The percentage decrease in glucose Rd did not differ between the groups. Adipose tissue insulin sensitivity (insulin-mediated suppression of plasma free fatty acids) decreased to the same extent after surgery in both groups. Rectal cancer surgery induced profound insulin resistance, affecting glucose and fatty acid metabolism. The preoperative nutrition supplement somewhat attenuated but did not prevent postoperative peripheral insulin resistance. The paper is published in the Journal of Parenteral and Enteral Nutrition.
May 2017:  Bile acids are established signaling molecules next to their role in the intestinal emulsification and uptake of lipids. Here, Thijs Pols aimed to identify a potential interaction between bile acids and CD4+ Th cells, which are central in adaptive immune responses. We screened distinct bile acid species for their potency to affect T cell function. Primary human and mouse CD4+ Th cells as well as Jurkat T cells were used to gain insight into the mechanism underlying these effects. We found that unconjugated lithocholic acid (LCA) impedes Th1 activation as measured by i) decreased production of the Th1 cytokines IFNγ and TNFαα, ii) decreased expression of the Th1 genes T-box protein expressed in T cells (T-bet), Stat-1 and Stat4, and iii) decreased STAT1α/β phosphorylation. Importantly, we observed that LCA impairs Th1 activation at physiological relevant concentrations. Profiling of MAPK signaling pathways in Jurkat T cells uncovered an inhibition of ERK-1/2 phosphorylation upon LCA exposure, which could provide an explanation for the impaired Th1 activation. LCA induces these effects via Vitamin D receptor (VDR) signaling since VDR RNA silencing abrogated these effects. These data reveal for the first time that LCA controls adaptive immunity via inhibition of Th1 activation. Many factors influence LCA levels, including bile acid-based drugs and gut microbiota. Our data may suggest that these factors also impact on adaptive immunity via a yet unrecognized LCA-Th cell axis. The paper is published in PLoS One.
Bile acids are established signaling molecules next to their role in the intestinal emulsification and uptake of lipids. Here, Thijs Pols aimed to identify a potential interaction between bile acids and CD4+ Th cells, which are central in adaptive immune responses. We screened distinct bile acid species for their potency to affect T cell function. Primary human and mouse CD4+ Th cells as well as Jurkat T cells were used to gain insight into the mechanism underlying these effects. We found that unconjugated lithocholic acid (LCA) impedes Th1 activation as measured by i) decreased production of the Th1 cytokines IFNγ and TNFαα, ii) decreased expression of the Th1 genes T-box protein expressed in T cells (T-bet), Stat-1 and Stat4, and iii) decreased STAT1α/β phosphorylation. Importantly, we observed that LCA impairs Th1 activation at physiological relevant concentrations. Profiling of MAPK signaling pathways in Jurkat T cells uncovered an inhibition of ERK-1/2 phosphorylation upon LCA exposure, which could provide an explanation for the impaired Th1 activation. LCA induces these effects via Vitamin D receptor (VDR) signaling since VDR RNA silencing abrogated these effects. These data reveal for the first time that LCA controls adaptive immunity via inhibition of Th1 activation. Many factors influence LCA levels, including bile acid-based drugs and gut microbiota. Our data may suggest that these factors also impact on adaptive immunity via a yet unrecognized LCA-Th cell axis. The paper is published in PLoS One.
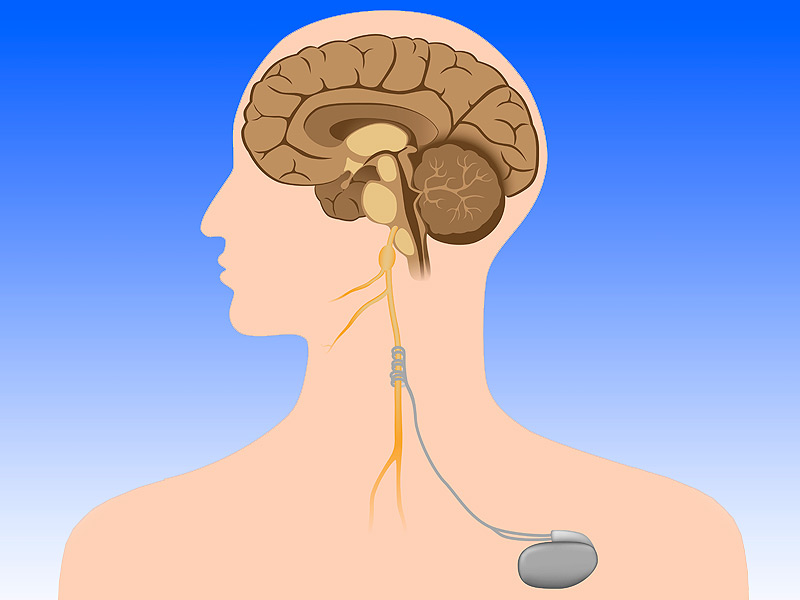 April 2017: Innervation by the autonomic nerve system might be involved in the regulation of many endocrine and metabolic processes and could therefore theoretically lead to unwanted side effects. Possible effects of VNS on secretion of hormones are currently unknown. Therefore, we evaluated the effects of a single VNS on plasma levels of pituitary hormones and parameters of postprandial metabolism. Man Wai Tang studied six female patients with RA twice in balanced assignment (crossover design) to either VNS or no stimulation. The patients selected for this substudy had been on VNS therapy daily for at least 3 months and at maximum of 24 months. We compared 10-, 20-, and 30-min poststimulus levels to baseline levels, and a 4-h mixed meal test was performed 30 min after VNS. We also determined energy expenditure (EE) by indirect calorimetry before and after VNS. VNS did not affect pituitary hormones (growth hormone, thyroid stimulating hormone, adrenocorticotropic hormone, prolactin, follicle-stimulating hormone, and luteinizing hormone), postprandial metabolism, or EE. Of note, VNS reduced early postprandial insulin secretion, but not AUC of postprandial plasma insulin levels. Cortisol and catecholamine levels in serum did not change significantly. Short stimulation of vagal activity by VNS reduces early postprandial insulin secretion, but not other hormone levels and postprandial response. Bile acid levels were unaffected. The paper is published in Clinical Rheumatology.
April 2017: Innervation by the autonomic nerve system might be involved in the regulation of many endocrine and metabolic processes and could therefore theoretically lead to unwanted side effects. Possible effects of VNS on secretion of hormones are currently unknown. Therefore, we evaluated the effects of a single VNS on plasma levels of pituitary hormones and parameters of postprandial metabolism. Man Wai Tang studied six female patients with RA twice in balanced assignment (crossover design) to either VNS or no stimulation. The patients selected for this substudy had been on VNS therapy daily for at least 3 months and at maximum of 24 months. We compared 10-, 20-, and 30-min poststimulus levels to baseline levels, and a 4-h mixed meal test was performed 30 min after VNS. We also determined energy expenditure (EE) by indirect calorimetry before and after VNS. VNS did not affect pituitary hormones (growth hormone, thyroid stimulating hormone, adrenocorticotropic hormone, prolactin, follicle-stimulating hormone, and luteinizing hormone), postprandial metabolism, or EE. Of note, VNS reduced early postprandial insulin secretion, but not AUC of postprandial plasma insulin levels. Cortisol and catecholamine levels in serum did not change significantly. Short stimulation of vagal activity by VNS reduces early postprandial insulin secretion, but not other hormone levels and postprandial response. Bile acid levels were unaffected. The paper is published in Clinical Rheumatology.
November 2016: We were very happy to contribute to a large study from Cambridge University (UK). Insulin resistance is a key mediator of obesity-related cardiometabolic disease, yet the mechanisms underlying this link remain obscure. Using an integrative genomic approach, Dr Luca Lotta from the MRC Epidemiology Unit in Cambridge identified 53 genomic regions associated with insulin resistance phenotypes (higher fasting insulin levels adjusted for BMI, lower HDL cholesterol levels and higher triglyceride levels) and provide evidence that their link with higher cardiometabolic risk is underpinned by an association with lower adipose mass in peripheral compartments. Using these 53 loci, we show a polygenic contribution to familial partial lipodystrophy type 1, a severe form of insulin resistance, and highlight shared molecular mechanisms in common/mild and rare/severe insulin resistance. Population-level genetic analyses combined with experiments in cellular models implicate CCDC92, DNAH10 and L3MBTL3 as previously unrecognized molecules influencing adipocyte differentiation. Moreover we used extreme phenotypes to validate our findings: here the Academical Medical Center contributed to this paper. Our findings support the notion that limited storage capacity of peripheral adipose tissue is an important etiological component in insulin-resistant cardiometabolic disease and highlight genes and mechanisms underpinning this link. The paper is published in Nature Genetics.
 October 2016: Samuel van Nierop shows the effect of a very-low-calorie diet on postprandial bile acid levels in the following paper. Bile acids (BA) are pleiotropic hormones affecting glucose and lipid metabolism. The physiochemical properties of different BA species affect their enterohepatic dynamics and their affinity for bile acid receptors. The BA pool composition is altered in patients with type 2 diabetes and obesity. In this study we used a 2-week very-low-calorie diet (VLCD) to investigate the effects of weight loss on BA pool composition and postprandial dynamics. We performed mixed meal tests in obese, insulin resistant subjects before and after the VLCD. We measured postprandial plasma levels of glucose, insulin, BA and the BA-induced enterokine fibroblast growth factor 19 (FGF19). The VLCD decreased weight by 4.5 ± 2.3 kg (p<0.0001) within 14 days. Weight loss increased peak postprandial deoxycholate (DCA) levels (median [IQR]: 0.90 [0.90] vs. 1.25 [1.35] µmol/L; p=0.045*). Other BA species, glucose, insulin and FGF19 levels and prandial excursions were not significantly affected. The VLCD decreased resting and postprandial energy expenditure by 7 and 11 % respectively. VLCD induced weight loss increased postprandial DCA peak levels and decreased resting energy expenditure in obese insulin resistant subjects. The paper is published in Clinical Nutrition.
October 2016: Samuel van Nierop shows the effect of a very-low-calorie diet on postprandial bile acid levels in the following paper. Bile acids (BA) are pleiotropic hormones affecting glucose and lipid metabolism. The physiochemical properties of different BA species affect their enterohepatic dynamics and their affinity for bile acid receptors. The BA pool composition is altered in patients with type 2 diabetes and obesity. In this study we used a 2-week very-low-calorie diet (VLCD) to investigate the effects of weight loss on BA pool composition and postprandial dynamics. We performed mixed meal tests in obese, insulin resistant subjects before and after the VLCD. We measured postprandial plasma levels of glucose, insulin, BA and the BA-induced enterokine fibroblast growth factor 19 (FGF19). The VLCD decreased weight by 4.5 ± 2.3 kg (p<0.0001) within 14 days. Weight loss increased peak postprandial deoxycholate (DCA) levels (median [IQR]: 0.90 [0.90] vs. 1.25 [1.35] µmol/L; p=0.045*). Other BA species, glucose, insulin and FGF19 levels and prandial excursions were not significantly affected. The VLCD decreased resting and postprandial energy expenditure by 7 and 11 % respectively. VLCD induced weight loss increased postprandial DCA peak levels and decreased resting energy expenditure in obese insulin resistant subjects. The paper is published in Clinical Nutrition.
September 2016: The bile acid receptor TGR5 (also  known as GPBAR1) is a promising target for the development of pharmacological interventions in metabolic diseases, including type 2 diabetes, obesity, and non-alcoholic steatohepatitis. We even think it is important for nutrition in general. As Sam van Nierop describes, TGR5 is expressed in many metabolically active tissues, but complex enterohepatic bile acid cycling limits the exposure of some of these tissues to the receptor ligand. Profound interspecies differences in the biology of bile acids and their receptors in different cells and tissues exist. Data from preclinical studies show promising effects of targeting TGR5 on outcomes such as weight loss, glucose metabolism, energy expenditure, and suppression of inflammation. However, clinical studies are scarce. We give a summary of key concepts in bile acid metabolism; outline different downstream effects of TGR5 activation; and review available data on TGR5 activation, with a focus on the translation of preclinical studies into clinically applicable findings. Studies in rodents suggest an important role for Tgr5 in Glp-1 secretion, insulin sensitivity, and energy expenditure. However, evidence of effects on these processes from human studies is less convincing. Ultimately, safe and selective human TGR5 agonists are needed to test the therapeutic potential of TGR5. The paper is published in the Lancet Endocrinology and Metabolism.
known as GPBAR1) is a promising target for the development of pharmacological interventions in metabolic diseases, including type 2 diabetes, obesity, and non-alcoholic steatohepatitis. We even think it is important for nutrition in general. As Sam van Nierop describes, TGR5 is expressed in many metabolically active tissues, but complex enterohepatic bile acid cycling limits the exposure of some of these tissues to the receptor ligand. Profound interspecies differences in the biology of bile acids and their receptors in different cells and tissues exist. Data from preclinical studies show promising effects of targeting TGR5 on outcomes such as weight loss, glucose metabolism, energy expenditure, and suppression of inflammation. However, clinical studies are scarce. We give a summary of key concepts in bile acid metabolism; outline different downstream effects of TGR5 activation; and review available data on TGR5 activation, with a focus on the translation of preclinical studies into clinically applicable findings. Studies in rodents suggest an important role for Tgr5 in Glp-1 secretion, insulin sensitivity, and energy expenditure. However, evidence of effects on these processes from human studies is less convincing. Ultimately, safe and selective human TGR5 agonists are needed to test the therapeutic potential of TGR5. The paper is published in the Lancet Endocrinology and Metabolism.
 July 2016: Acylcarnitines, fatty acid oxidation (FAO) intermediates, have been implicated in diet-induced insulin resistance and type 2 diabetes mellitus, as increased levels are found in obese insulin resistant humans. Moreover plasma acylcarnitines havebeen associated with clinical parameters related to glucose metabolism, such as fasting glucose levels and HbA1c. Marieke Schooneman hypothesized that plasma acylcarnitines would correlate with energy expenditure, insulin sensitivity and other clinical parameters before and during a weight loss intervention. In close collaboration with the laboratory of Professor Vidal-Puig in the Instistute of Metabolic Science in Cambridge (UK) and Sander Houten from Mount Sinai in New York (U.S.), we measured plasma acylcarnitines in 60 obese subjects before and after a 12 week weight loss intervention. These samples originated from three different interventions (diet alone (n = 20); diet and exercise (n = 21); diet and drug treatment (n = 19)). Acylcarnitine profiles were analysed in relation to clinical parameters of glucose metabolism, insulin sensitivity and energy expenditure. Conclusions were drawn from all 60 subjects together. Despite amelioration of HOMA-IR, plasma acylcarnitines levels increased during weight loss. HOMA-IR, energy expenditure and respiratory exchange ratio were not related to plasma acylcarnitines. However non-esterified fatty acids correlated strongly with several acylcarnitines at baseline and during the weight loss intervention (p < 0.001). Acylcarnitines did not correlate with clinical parameters of glucose metabolism during weight loss, questioning their role in insulin resistance and type 2 diabetes mellitus. The paper is published in the Archives of Biochemistry and Biophysics.
July 2016: Acylcarnitines, fatty acid oxidation (FAO) intermediates, have been implicated in diet-induced insulin resistance and type 2 diabetes mellitus, as increased levels are found in obese insulin resistant humans. Moreover plasma acylcarnitines havebeen associated with clinical parameters related to glucose metabolism, such as fasting glucose levels and HbA1c. Marieke Schooneman hypothesized that plasma acylcarnitines would correlate with energy expenditure, insulin sensitivity and other clinical parameters before and during a weight loss intervention. In close collaboration with the laboratory of Professor Vidal-Puig in the Instistute of Metabolic Science in Cambridge (UK) and Sander Houten from Mount Sinai in New York (U.S.), we measured plasma acylcarnitines in 60 obese subjects before and after a 12 week weight loss intervention. These samples originated from three different interventions (diet alone (n = 20); diet and exercise (n = 21); diet and drug treatment (n = 19)). Acylcarnitine profiles were analysed in relation to clinical parameters of glucose metabolism, insulin sensitivity and energy expenditure. Conclusions were drawn from all 60 subjects together. Despite amelioration of HOMA-IR, plasma acylcarnitines levels increased during weight loss. HOMA-IR, energy expenditure and respiratory exchange ratio were not related to plasma acylcarnitines. However non-esterified fatty acids correlated strongly with several acylcarnitines at baseline and during the weight loss intervention (p < 0.001). Acylcarnitines did not correlate with clinical parameters of glucose metabolism during weight loss, questioning their role in insulin resistance and type 2 diabetes mellitus. The paper is published in the Archives of Biochemistry and Biophysics.
 July 2016: Lonneke Bahler works her way through brown adipose tissue again! Bromocriptine is a centrally acting dopamine receptor agonist that improves insulin sensitivity in obese subjects. No explanation has been found for this effect of bromocriptine. Brown adipose tissue (BAT) expends energy via heat production and might be a mediator of the insulin sensitizing effect of bromocriptine. Since the central sympathetic nervous system is the primary activator of BAT, we hypothesized that dopamine plays a role in the activation of BAT. We included 8 lean, healthy Caucasian males. All subjects were studied before and after using bromocriptine. On these 2 study visits we measured metabolic BAT activity, defined as maximal standardized uptake value (SUVmax), using 18F-Fluorodeoxyglucose Positron Emission Tomography CT scans. Furthermore we investigated glucose metabolism with a 7 point oral glucose tolerance test, energy expenditure (EE) using indirect calorimetry. The use of bromocriptine did not significantly alter metabolic BAT activity and unexpectedly, subjects became significantly less insulin sensitive when using bromocriptine. The area under the curve for glucose increased after bromocriptin, but the area under the curve for insulin also increased. Bromocriptine administration does not activate BAT and does not increase EE but decreases insulin sensitivity in lean, healthy males. The paper is published in Diabetes and Metabolism.
July 2016: Lonneke Bahler works her way through brown adipose tissue again! Bromocriptine is a centrally acting dopamine receptor agonist that improves insulin sensitivity in obese subjects. No explanation has been found for this effect of bromocriptine. Brown adipose tissue (BAT) expends energy via heat production and might be a mediator of the insulin sensitizing effect of bromocriptine. Since the central sympathetic nervous system is the primary activator of BAT, we hypothesized that dopamine plays a role in the activation of BAT. We included 8 lean, healthy Caucasian males. All subjects were studied before and after using bromocriptine. On these 2 study visits we measured metabolic BAT activity, defined as maximal standardized uptake value (SUVmax), using 18F-Fluorodeoxyglucose Positron Emission Tomography CT scans. Furthermore we investigated glucose metabolism with a 7 point oral glucose tolerance test, energy expenditure (EE) using indirect calorimetry. The use of bromocriptine did not significantly alter metabolic BAT activity and unexpectedly, subjects became significantly less insulin sensitive when using bromocriptine. The area under the curve for glucose increased after bromocriptin, but the area under the curve for insulin also increased. Bromocriptine administration does not activate BAT and does not increase EE but decreases insulin sensitivity in lean, healthy males. The paper is published in Diabetes and Metabolism.
 June 2016: We are happy and proud that we could collaborate in a bile acid project of Dr. Filip Knop from Copenhagen. Bile acids exert regulatory effects on lipid and carbohydrate metabolism by interaction with membrane or intracellular proteins including the nuclear farnesoid X receptor (FXR) in the gastrointestinal tract and the liver. FXR activation in the ileum leads to secretion of fibroblast growth factor 19 (FGF-19), a gut hormone, which may be implicated in postprandial glucose metabolism. However, individual bile acids exhibit dissimilar binding affinity and efficacy towards FXR.In this study, we characterized postprandial concentrations of individual bile acids and FGF-19 in plasma from 15 patients with type 2 diabetes (T2D) and 15 healthy age, gender and body mass index-matched controls with normal glucose tolerance (NGT) undergoing 4 separate ‘meal’ tests: a 75g-oral glucose tolerance test (OGTT) and 3 isocaloric and isovolemic liquid meals with low, medium and high fat content, respectively. We found that total bile acid concentrations increased with increasing meal fat content (P<0.05), peaked after 1-2h and were higher in T2D patients vs. controls (OGTT, low and medium fat meals (P<0.05); high fat meal (P=0.30)). Differences reflected mainly unconjugated and glycine-conjugated forms of deoxycholic acid (DCA) and to a lesser extent cholic acid (CA) and ursodeoxycholic acid (UDCA), whereas chenodeoxycholic acid (CDCA) concentrations were comparable in the two groups. A tendency to lower FGF-19 concentrations was observed in T2D patients, but differences were not statistically significant due to considerable variation. In conclusion, postprandial plasma patterns of bile acids with FXR agonistic (CDCA, DCA and CA) and FXR antagonistic properties (UDCA) in T2D patients support the notion of a ‘T2D-bile acid-FGF-19’ phenotype with possible pathophysiological implications. David Sonne is the first author and the paper is published in the Journal of Clinical Endocrinology and Metabolism!
June 2016: We are happy and proud that we could collaborate in a bile acid project of Dr. Filip Knop from Copenhagen. Bile acids exert regulatory effects on lipid and carbohydrate metabolism by interaction with membrane or intracellular proteins including the nuclear farnesoid X receptor (FXR) in the gastrointestinal tract and the liver. FXR activation in the ileum leads to secretion of fibroblast growth factor 19 (FGF-19), a gut hormone, which may be implicated in postprandial glucose metabolism. However, individual bile acids exhibit dissimilar binding affinity and efficacy towards FXR.In this study, we characterized postprandial concentrations of individual bile acids and FGF-19 in plasma from 15 patients with type 2 diabetes (T2D) and 15 healthy age, gender and body mass index-matched controls with normal glucose tolerance (NGT) undergoing 4 separate ‘meal’ tests: a 75g-oral glucose tolerance test (OGTT) and 3 isocaloric and isovolemic liquid meals with low, medium and high fat content, respectively. We found that total bile acid concentrations increased with increasing meal fat content (P<0.05), peaked after 1-2h and were higher in T2D patients vs. controls (OGTT, low and medium fat meals (P<0.05); high fat meal (P=0.30)). Differences reflected mainly unconjugated and glycine-conjugated forms of deoxycholic acid (DCA) and to a lesser extent cholic acid (CA) and ursodeoxycholic acid (UDCA), whereas chenodeoxycholic acid (CDCA) concentrations were comparable in the two groups. A tendency to lower FGF-19 concentrations was observed in T2D patients, but differences were not statistically significant due to considerable variation. In conclusion, postprandial plasma patterns of bile acids with FXR agonistic (CDCA, DCA and CA) and FXR antagonistic properties (UDCA) in T2D patients support the notion of a ‘T2D-bile acid-FGF-19’ phenotype with possible pathophysiological implications. David Sonne is the first author and the paper is published in the Journal of Clinical Endocrinology and Metabolism!
 May 2016: The way you eat, or don’t eat, influences how you metabolize your pills as Roos Achterbergh shows in our paper. Her study assesses the effects of a short-term hypercaloric high fat diet on metabolism of five oral drugs, which are each specific for a single P450 isoform: midazolam (CYP3A4), omeprazole (CYP2C19), metoprolol (CYP2D6), S-warfarin (CYP2C9) and caffeine (CYP1A2). Methods: In 9 healthy volunteers, pharmacokinetics of the five drugs were assessed after an overnight fast at two separate occasions: after a regular diet and after 3 days of a hypercaloric high fat diet (i.e. regular diet supplemented with 500 mL cream [1715 kcal, 35% fat]). Pharmacokinetic parameters (mean [SEM]) were estimated by non-compartmental analysis. Results: The high fat diet increased exposure to midazolam by 19% from 24.7 (2.6) to 29.5 (3.6) ng ml-1h-1 (p=0.04) and exposure to omeprazole by 31 % from 726 (104) to 951 (168) ng ml-1h-1 (p=0.05). Exposure to metoprolol, caffeine and S-warfarin was not affected by the high fat diet. Conclusion: A short-term hypercaloric high fat diet increases exposure to midazolam and omeprazole, possibly reflecting modulation of CYP3A4 and CYP2C19. The paper is online here.
May 2016: The way you eat, or don’t eat, influences how you metabolize your pills as Roos Achterbergh shows in our paper. Her study assesses the effects of a short-term hypercaloric high fat diet on metabolism of five oral drugs, which are each specific for a single P450 isoform: midazolam (CYP3A4), omeprazole (CYP2C19), metoprolol (CYP2D6), S-warfarin (CYP2C9) and caffeine (CYP1A2). Methods: In 9 healthy volunteers, pharmacokinetics of the five drugs were assessed after an overnight fast at two separate occasions: after a regular diet and after 3 days of a hypercaloric high fat diet (i.e. regular diet supplemented with 500 mL cream [1715 kcal, 35% fat]). Pharmacokinetic parameters (mean [SEM]) were estimated by non-compartmental analysis. Results: The high fat diet increased exposure to midazolam by 19% from 24.7 (2.6) to 29.5 (3.6) ng ml-1h-1 (p=0.04) and exposure to omeprazole by 31 % from 726 (104) to 951 (168) ng ml-1h-1 (p=0.05). Exposure to metoprolol, caffeine and S-warfarin was not affected by the high fat diet. Conclusion: A short-term hypercaloric high fat diet increases exposure to midazolam and omeprazole, possibly reflecting modulation of CYP3A4 and CYP2C19. The paper is online here.
Ap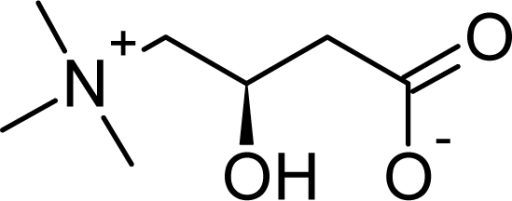 ril 2016: Marieke Schooneman shows in our new paper “The impact of altered carnitine availability on acylcarnitine metabolism, energy expenditure and glucose tolerance in diet-induced obese mice” how carnitine affects acylcarnitine profiles and glucose metabolism. We hypothesized that increasing free carnitine levels by administration of the carnitine precursor γ-butyrobetaine (γBB) could facilitate FAO, thereby improving insulin sensitivity. However, mice on a high fat diet with γBB supplementation were not protected against weight gain in spite of marked alterations in the acylcarnitine profiles in plasma and liver. Altogether, increasing carnitine availability affects acylcarnitine profiles in plasma and liver but does not modulate glucose tolerance or insulin sensitivity. This may be due to the lacking effect on muscle acylcarnitine profiles, as muscle tissue is an important contributor to whole body insulin sensitivity. These results warrant caution on making associations between plasma acylcarnitine levels and insulin resistance. The paper is published in Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease.
ril 2016: Marieke Schooneman shows in our new paper “The impact of altered carnitine availability on acylcarnitine metabolism, energy expenditure and glucose tolerance in diet-induced obese mice” how carnitine affects acylcarnitine profiles and glucose metabolism. We hypothesized that increasing free carnitine levels by administration of the carnitine precursor γ-butyrobetaine (γBB) could facilitate FAO, thereby improving insulin sensitivity. However, mice on a high fat diet with γBB supplementation were not protected against weight gain in spite of marked alterations in the acylcarnitine profiles in plasma and liver. Altogether, increasing carnitine availability affects acylcarnitine profiles in plasma and liver but does not modulate glucose tolerance or insulin sensitivity. This may be due to the lacking effect on muscle acylcarnitine profiles, as muscle tissue is an important contributor to whole body insulin sensitivity. These results warrant caution on making associations between plasma acylcarnitine levels and insulin resistance. The paper is published in Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease.
February 2016: Kasper ter Horst publishes  another paper on the primary aspects of insulin resistance in obesity. He aimed to determine the contribution of these processes to impaired fasting glucose (IFG) levels in obese non-diabetic adults. Therefore 131 obese non-diabetic adults with normal fasting glucose levels were included (NFG; fasting glucose <5.6 mmol/l; 62 men, 25 women; mean±SEM age 49±1 y; median (IQR) BMI 36 (34-41) kg/m2) or IFG (fasting glucose 5.6-6.9 mmol/l; 35 men, 9 women; age 53±1 y; BMI 36 (34-42) kg/m2) and studied basal EGP and hepatic, adipose tissue and peripheral insulin sensitivity by two-step euglycemic hyperinsulinemic clamp studies with [6,6-2H2]glucose infusion. Compared to equally obese adults with NFG, individuals with IFG did not differ in basal EGP (9.1±0.2 vs 9.8±0.3 µmol·kg-1·min-1, p=0.082), insulin-mediated suppression of circulating free fatty acid levels (75±1 vs 72±3%, p=0.240) and insulin-stimulated glucose disposal (26.6±1.0 vs 25.2±1.5 µmol·kg-1·min-1, p=0.441). Insulin-mediated suppression of EGP (68±2 vs 55±3%, p<0.001) was markedly reduced in obese subjects with IFG. So, hepatic insulin resistance is a distinct metabolic feature of IFG in obesity and this is not the case for insulin sensitivity of free fatty acid suppression and skeletal muscle. Here liver seems to play a central role. Impaired insulin action in the liver, but not in adipose tissue or muscle, is a distinct metabolic feature of impaired fasting glucose in obese humans. The paper is published in Metabolism.
another paper on the primary aspects of insulin resistance in obesity. He aimed to determine the contribution of these processes to impaired fasting glucose (IFG) levels in obese non-diabetic adults. Therefore 131 obese non-diabetic adults with normal fasting glucose levels were included (NFG; fasting glucose <5.6 mmol/l; 62 men, 25 women; mean±SEM age 49±1 y; median (IQR) BMI 36 (34-41) kg/m2) or IFG (fasting glucose 5.6-6.9 mmol/l; 35 men, 9 women; age 53±1 y; BMI 36 (34-42) kg/m2) and studied basal EGP and hepatic, adipose tissue and peripheral insulin sensitivity by two-step euglycemic hyperinsulinemic clamp studies with [6,6-2H2]glucose infusion. Compared to equally obese adults with NFG, individuals with IFG did not differ in basal EGP (9.1±0.2 vs 9.8±0.3 µmol·kg-1·min-1, p=0.082), insulin-mediated suppression of circulating free fatty acid levels (75±1 vs 72±3%, p=0.240) and insulin-stimulated glucose disposal (26.6±1.0 vs 25.2±1.5 µmol·kg-1·min-1, p=0.441). Insulin-mediated suppression of EGP (68±2 vs 55±3%, p<0.001) was markedly reduced in obese subjects with IFG. So, hepatic insulin resistance is a distinct metabolic feature of IFG in obesity and this is not the case for insulin sensitivity of free fatty acid suppression and skeletal muscle. Here liver seems to play a central role. Impaired insulin action in the liver, but not in adipose tissue or muscle, is a distinct metabolic feature of impaired fasting glucose in obese humans. The paper is published in Metabolism.
 February 2016: We love the cold!! Yes we really do! The Professor Vidal-Puig Lab performed a cold exposure study since mild cold exposure increases energy expenditure (EE) and can influence energy balance. Surely if hunger and food intake do not increase at the same time. We exposed healthy volunteers to cold and thermoneutrality in the Clinical Research Facility in Addenbrookes Hospital in Cambridge. We measured hunger sensation and actual free food intake. Also, we assessed thermal comfort and skin temperatures changes by infrared thermography. Five males and five females were exposed to either 18 degrees centigrade (mild cold) or 24 degrees for 2.5 hours. Metabolic rate, vital signs, skin temperature, blood biochemistry, cold and hunger scores were all measured. Finally, this was followed by an ad libitum meal to obtain actual desired energy intake after cold exposure. We could replicate the cold induced increase in EE. But no differences in hunger, food intake or satiety were detected. After longer cold exposure, high cold sensation scores were reported, which were negatively correlated to thermogenesis. Skin temperature in the sternal area was tightly correlated to the increase in energy expenditure. So, short-term mild cold exposure increases EE but no changes in food intake. Mild cold exposure resulted in significant thermal discomfort, which was negatively correlated to the increase in energy expenditure. These data provide further insight on cold exposure as an anti-obesity measure. The paper is published in Endocrine Connections.
February 2016: We love the cold!! Yes we really do! The Professor Vidal-Puig Lab performed a cold exposure study since mild cold exposure increases energy expenditure (EE) and can influence energy balance. Surely if hunger and food intake do not increase at the same time. We exposed healthy volunteers to cold and thermoneutrality in the Clinical Research Facility in Addenbrookes Hospital in Cambridge. We measured hunger sensation and actual free food intake. Also, we assessed thermal comfort and skin temperatures changes by infrared thermography. Five males and five females were exposed to either 18 degrees centigrade (mild cold) or 24 degrees for 2.5 hours. Metabolic rate, vital signs, skin temperature, blood biochemistry, cold and hunger scores were all measured. Finally, this was followed by an ad libitum meal to obtain actual desired energy intake after cold exposure. We could replicate the cold induced increase in EE. But no differences in hunger, food intake or satiety were detected. After longer cold exposure, high cold sensation scores were reported, which were negatively correlated to thermogenesis. Skin temperature in the sternal area was tightly correlated to the increase in energy expenditure. So, short-term mild cold exposure increases EE but no changes in food intake. Mild cold exposure resulted in significant thermal discomfort, which was negatively correlated to the increase in energy expenditure. These data provide further insight on cold exposure as an anti-obesity measure. The paper is published in Endocrine Connections.
November 2015: Lonneke Bahler shows new insights on brown adipose tissue (BAT). This tissue could facilitate weight ![]() loss by increasing energy expenditure. Cold is a potent stimulator of BAT, activating BAT primarily through the sympathetic nervous system (SNS). Older or overweight individuals have less metabolic BAT activity than the lean and young, but the role of the SNS in this decline is unknown. Lonneke aimed to determine whether this lower metabolic BAT activity in older or overweight individuals can be explained by a lower SNS response to cold. SUVmax, BAT volume and SQUVmax were significantly different between young and old (SUVmax 7.9[4.2-17.3] vs. 2.9[0.0-4.0], volume 124.8[10.9-338.8] vs 3.4 [0.0-10.9] and SQUVmax 2.7[1.9-4.7] vs 0.0[0.0-2.2] all p<0.01) but not between lean and obese (SUVmax 7.9[4.2-17.3] vs 4.0[0.0-13.5] P = 0.69; volume 124.8[10.9-338.8] vs 11.8 [0.0-190.2] P = 0.64 and SQUVmax 2.7[1.9-4.7]vs 1.7[0-3.5] P = 0.69). Both sympathetic drive and BAT activity are lower in older but not in obese males. This may have consequences for autonomic BAT regulation in obesity. Bahler L, Verberne HJ, Admiraal W, Stok WJ, Soeters MR, Hoekstra JB, et al. Differences in Sympathetic Nervous Stimulation of Brown Adipose tissue between the young and old and the lean and obese. J Nucl Med. 2015 Nov 25.
loss by increasing energy expenditure. Cold is a potent stimulator of BAT, activating BAT primarily through the sympathetic nervous system (SNS). Older or overweight individuals have less metabolic BAT activity than the lean and young, but the role of the SNS in this decline is unknown. Lonneke aimed to determine whether this lower metabolic BAT activity in older or overweight individuals can be explained by a lower SNS response to cold. SUVmax, BAT volume and SQUVmax were significantly different between young and old (SUVmax 7.9[4.2-17.3] vs. 2.9[0.0-4.0], volume 124.8[10.9-338.8] vs 3.4 [0.0-10.9] and SQUVmax 2.7[1.9-4.7] vs 0.0[0.0-2.2] all p<0.01) but not between lean and obese (SUVmax 7.9[4.2-17.3] vs 4.0[0.0-13.5] P = 0.69; volume 124.8[10.9-338.8] vs 11.8 [0.0-190.2] P = 0.64 and SQUVmax 2.7[1.9-4.7]vs 1.7[0-3.5] P = 0.69). Both sympathetic drive and BAT activity are lower in older but not in obese males. This may have consequences for autonomic BAT regulation in obesity. Bahler L, Verberne HJ, Admiraal W, Stok WJ, Soeters MR, Hoekstra JB, et al. Differences in Sympathetic Nervous Stimulation of Brown Adipose tissue between the young and old and the lean and obese. J Nucl Med. 2015 Nov 25.
 September 2015: In the recent paper by Marieke Schooneman (American Journal of Physiology – Endocrinology and Metabolism Published 1 August 2015 Vol. 309 no. 3, E256-E264), we show that the liver has a key role in acylcarnitine metabolism, with high net fluxes of C2-carnitine both in the fasted and fed state, whereas the contribution of skeletal muscle is minor. Acylcarnitines are derived from mitochondrial acyl-CoA metabolism and have been associated with diet-induced insulin resistance. However, plasma acylcarnitine profiles have been shown to poorly reflect whole body acylcarnitine metabolism. We aimed to clarify the individual role of different organ compartments in whole body acylcarnitine metabolism in twelve cross-bred pigs with intravascular catheters were positioned before and after the liver, gut, hindquarter muscle compartment, and kidney. Before and after a mixed meal, we measured acylcarnitine profiles at several time points and calculated net transorgan acylcarnitine fluxes. Fasting plasma acylcarnitine concentrations correlated with net hepatic transorgan fluxes of free and C2- and C16-carnitine. Transorgan acylcarnitine fluxes were small, except for a pronounced net hepatic C2-carnitine production. The peak of the postprandial acylcarnitine fluxes was between 60 and 90 min. Acylcarnitine production or release was seen in the gut and liver and consisted mostly of C2-carnitine. Acylcarnitines were extracted by the kidney. No significant net muscle acylcarnitine flux was observed. These results further clarify the role of different organ compartments in the metabolism of different acylcarnitine species. This research was done in close collaboration with Professor Mick Deutz, Department of Health & Kinesiology, Director of the Center for Translational Research in Aging & Longevity, Texas A&M University.
September 2015: In the recent paper by Marieke Schooneman (American Journal of Physiology – Endocrinology and Metabolism Published 1 August 2015 Vol. 309 no. 3, E256-E264), we show that the liver has a key role in acylcarnitine metabolism, with high net fluxes of C2-carnitine both in the fasted and fed state, whereas the contribution of skeletal muscle is minor. Acylcarnitines are derived from mitochondrial acyl-CoA metabolism and have been associated with diet-induced insulin resistance. However, plasma acylcarnitine profiles have been shown to poorly reflect whole body acylcarnitine metabolism. We aimed to clarify the individual role of different organ compartments in whole body acylcarnitine metabolism in twelve cross-bred pigs with intravascular catheters were positioned before and after the liver, gut, hindquarter muscle compartment, and kidney. Before and after a mixed meal, we measured acylcarnitine profiles at several time points and calculated net transorgan acylcarnitine fluxes. Fasting plasma acylcarnitine concentrations correlated with net hepatic transorgan fluxes of free and C2- and C16-carnitine. Transorgan acylcarnitine fluxes were small, except for a pronounced net hepatic C2-carnitine production. The peak of the postprandial acylcarnitine fluxes was between 60 and 90 min. Acylcarnitine production or release was seen in the gut and liver and consisted mostly of C2-carnitine. Acylcarnitines were extracted by the kidney. No significant net muscle acylcarnitine flux was observed. These results further clarify the role of different organ compartments in the metabolism of different acylcarnitine species. This research was done in close collaboration with Professor Mick Deutz, Department of Health & Kinesiology, Director of the Center for Translational Research in Aging & Longevity, Texas A&M University.
August 2015: Kasper ter Horst shows this summer that fasting insulin may be used for identification of insulin-resistant (or metabolically unhealthy)obese men in research and clinical settings (International Journal of Obesity advance online publication 4 August 2015; doi: 10.1038/ijo.2015.125). Indeed, insulin resistance is the major contributor to cardiometabolic complications of obesity. Here we aimed to (1) establish cutoff points for insulin resistance from euglycemic hyperinsulinemic clamps (EHCs), (2) identify insulin-resistant obese subjects and (3) predict insulin resistance from routinely measured variables. Data was assembled from non-obese (n=112) and obese (n=100) men who underwent two-step clamps using [6,6-2H2]glucose as tracer (insulin infusion dose 20 and 60 mU m-2 min-1, respectively). Reference ranges for hepatic and peripheral insulin sensitivity were calculated from healthy non-obese men. Based on these reference values, obese men with preserved insulin sensitivity or insulin resistance were identified. Most obese men have hepatic insulin sensitivity within the range of non-obese controls, but below-normal peripheral insulin sensitivity, that is, insulin resistance.
insulin-resistant (or metabolically unhealthy)obese men in research and clinical settings (International Journal of Obesity advance online publication 4 August 2015; doi: 10.1038/ijo.2015.125). Indeed, insulin resistance is the major contributor to cardiometabolic complications of obesity. Here we aimed to (1) establish cutoff points for insulin resistance from euglycemic hyperinsulinemic clamps (EHCs), (2) identify insulin-resistant obese subjects and (3) predict insulin resistance from routinely measured variables. Data was assembled from non-obese (n=112) and obese (n=100) men who underwent two-step clamps using [6,6-2H2]glucose as tracer (insulin infusion dose 20 and 60 mU m-2 min-1, respectively). Reference ranges for hepatic and peripheral insulin sensitivity were calculated from healthy non-obese men. Based on these reference values, obese men with preserved insulin sensitivity or insulin resistance were identified. Most obese men have hepatic insulin sensitivity within the range of non-obese controls, but below-normal peripheral insulin sensitivity, that is, insulin resistance.
Hannah Eggink Published a review paper on the role of the bile acid receptor TGR5 in inflammation early 2015. This is a G protein-coupled receptor that is best known for its activation by bile acids. TGR5 has been found to regulate a number of specific processes, including energy expenditure and glucagon-like peptide-1 release. Other actions in which TGR5 is implied range from regulating bile acid homeostasis to playing a role in the nervous system. The receptor is increasingly associated with the regulation of inflammatory responses in a number of cells that are relevant to the immune response. TGR5 exerts antiinflammatory actions by decreasing adhesion molecule expression in endothelial cells and inhibiting proinflammatory cytokine production in macrophages. A number of animal models also hint toward the antiinflammatory actions of TGR5. These include models of atherosclerosis, colitis, and inflammation-driven liver diseases. In the current review, we provide a comprehensive overview of TGR5 with a focus on its role in inflammation. We furthermore describe the currently known agonists of TGR5 and discuss some of the recent findings on TGR5 signaling. The potential drawbacks, as well as the encouraging prospects, of TGR5 will be discussed in view of TGR5 as a therapeutic target in diseases with inflammatory facets. Hannah Eggink, Maarten Soeters and Thijs Pols. TGR5 ligands as potential therapeutics in inflammatory diseases. Int J Interf Cytokine Mediat Res. 2014;6(1):27–38.